Team:Potsdam Bioware/Project/Details Microviridin
From 2011.igem.org
Contents |
Microviridin
Introduction
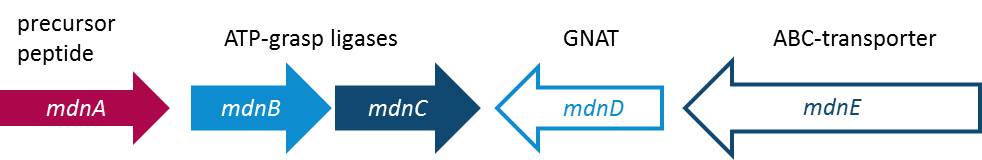
Cyanobacteria are known as the blue-green-algae because their living space is water and their feeding mechanism is photosynthetic. The Cyanobacteria are know for some special and unique metabolites. Some of these metabolites are part of the microviridin family. Special attributes of microviridins are their occurrence as tricyclic depsipeptides, means peptides whose one or more amide-bond is replaced by an ester-bond, unparallel cage-like architecture and their ability to inhibit several proteases. Microviridin B for example is told to function as an elastase inhibitor what would be an therapeutic attempt according to fix the out of control gone function of elastase in lung emphysema. In such a case it is beyond debate how important it is to figure out the biosynthesis of this peptide. The research group of Prof. Dittmann at the University of Potsdam was successful and able to report about the composition of the gene cluster from Micocystis aeruginosa NIES298 expressing Microviridin B. Moreover they have been convinced that there is existing one unique biosynthetic mechanism for microviridins in Microcystis strains. Unusual for depsipeptides they discovered that they are ribosomal synthesized. The mdn gene cluster is build up of a gene mdnA, encoding for the putative precursor peptide of the microvirdin, two genes encoding ATP-grasp-type ligases mdnB and mdnC, an ABC transporter encoding gene mdnE as well as one encoding an N-acetyltransferase of the GNAT family mdnD (Ziemert et al., 2008). Moreover in their recent studies they report about the discovery of an existing natural diversity of microviridin precursors genes in set of closely related Mycrocystis laboratory strains. This furthermore would mean and lead to a heterologous production of novel microviridins in E. coli. To give evidence of their discovery they identified and characterized a new Microviridin L from the strain Mycrocystis aeruginosa NIES843 (Ziemert et al., 2010). All in all the microviridins, the tricyclic depsipeptide are build up through a gene cluster of five mdn-components each fulfill one function to make the hole cluster functional. This showed the team of Prof. Dr. Dittmann as the peptide is only correctly processed in E. coli if the entire cluster mdnABCDE is expressed. In addition the new discovery is promising a small flexibility of the microviridin ligases as an open window for the size of the natural microviridin library and thus microviridin functions and hence for example therapeutic benefit that needs to be discovered. The following illustration is going to give you a better overview (Fig. 1).
Modularization of the mdn gene cluster
Using two vectors (pARW071 and pARW089, respectively) containing the mdn biosynthetic gene cluster, we designed PCR primers for the amplification of the mdn genes. Using these primers, we obtained the gene fragments of mdnA, mdnB, mdnC, mdnD, mdnE and for the whole cluster, respectively. Due to the sophisticated incorporation of the sequences of the iGEM restriction enzymes into the primer sequence our BioBricks comply with the BioBrick standards.
The following image (Fig. 2) shows the generic construction of the BioBrick carrying the mdnA gene.
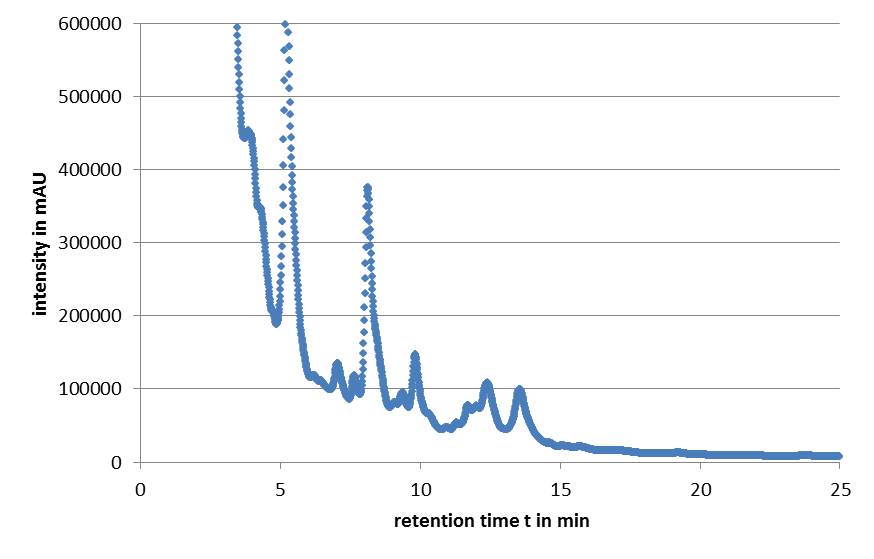
Microviridin production in E. coli cells expressing the mdn genes was monitored by reverse phase HPLC. Qualitative analysis involves running a standard that contains the target analytes. The vectors pARW071 and pARW089 served as a control in our experiments because these vectors contain the original mdn-cluster. We could use the retention time as a way to determine the presence of the microviridin production in other samples. HPLC analysis of the purified compound yielded a high peak with a retention time of approximately 5 min. Minor peaks could be detected during the following 7 min (Fig. 3).
All HPLC chromatograms of the isolated mdnA showed reliable peaks with a retention time of 5 min together with a number of following minor peaks.
Fractions of the peaks were sampled and the identity of microviridin and also the presence of cyclization, which is important for the activity, was affirmed with mass spectrometry (Fig. 4). By using a mass spectrometer it is possible to determine both the elemental composition of a fraction and the chemical structure of molecules.
Generating mdnA gene libraries
In searching for optimized and novel microviridin peptides, libraries of mutated microviridins were established. Therefore a number of sites in the amino acid sequence of the precursor peptide (MdnA) were chosen.
In Microcystis, microviridins are synthesized from a ribosomal precursor peptide (MdnA). Therefore the microviridin gene cluster is necessary. This 6.5 kb biosynthesis gene cluster includes two genes (mdnB and mdnC), which encodes ATP-grasp-type ligases and further the mdnE gene encoding an ABC transporter as well as mdnD gene encoding a N-acetyltransferase (Ziemert et al., 2008). In consequence the biosynthesis gene cluster genes mdnB, mdnC, mdnD and mdnE cannot be mutated. Otherwise only misprocessed and non-function variants of microviridin occur (Ziemert et al., 2008). Thus merely the modification of the mdnA gene can lead to effective changes in the microviridin structure and function.

The precursor peptide MdnA consists of a leader peptide and a core peptide (Fig. 5). Ziemert et al. (2008) has shown that the N-terminal leader peptide contains highly conserved double glycine motifs (Ziemert et al. 2008). However remarkable variations were found in region, which encodes the 14-amino acid sequence of the C-terminal microviridin core peptide (Ziemert et al. 2010). Consequently, mutations in the MdnA core peptide seem to be the most promising option for modification of microviridin. For cyclization, the amino acid side chains of the core peptide form omega-ester and omega-amino bonds. One loop is formed between threonine and aspartic acid, the second loop between lysine and aspartic acid and the third loop between serine and glutamic acid (Fig. 5). The sequence of this loop forming amino acids constitute an exception while mutation of the mdnA gene (Ziemert et al., 2008 and 2010).
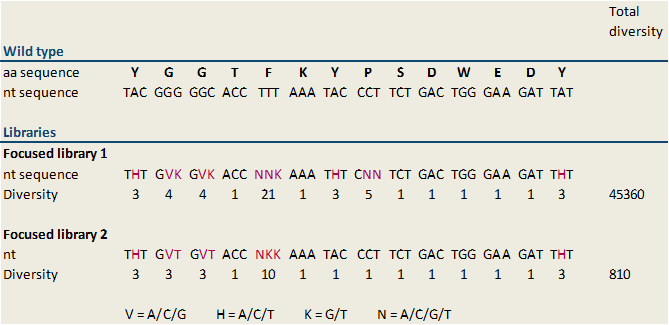
To find optimized and novel microviridin variants, several libraries carrying modifications in the sequence of the MdnA core peptide were established. These libraries were generated by randomized oligonucleotide synthesis. Therefore a forward oligonucleotide displaying a part of conserved leader peptide sequence was built. Further a reverse oligonucleotide was created. The first part of this oligonucleotide is 20 bases, which overlap with the forward oligonucleotide and which is necessary for hybridization of both, forward and reverse, oligonucleotides. In the middle of the sequence modified bases were inserted. For cloning, the forward oligonucleotide starts with the blunt restricted site of SfoI. This results therein that the oligonucleotide can ligate in the SfoI restricted vector without previous digest of the oligonucleotide. The last part of the reverse oligonucleotides displays an AatII restriction site for cloning. Digestion with AatII results in sticky ends, thus wrong way round insertion of the oligonucleotide is impossible. Forward and reverse oligonucleotides were combined by performing a fill-in reaction. Because we designed several libraries with different diversity rates, the modified bases, which are inserted in the middle of the reverse primer, vary. All of our generated libraries contain mutations of the sequence of the MdnA core peptide at several sites, but not at the loop forming sites. Thus we are thinking that cyclization of microviridin happens accurately. A library with a diversity of 45,360 (focused library 1) and a second focused library showing a minor diversity of 810 (focused library 2) were designed. The included sequence modifications plus the diversity of our libraries are shown in figure 6. In our further studies we worked on focused library 2 primarily. In this library the nucleotide sequences encoding glycine residues were changed in GVK in all cases (Figure 6). GVK stands for guanine at the first position, adenine, cytosine or guanine at the second position and further thymine at the third position of the codon. These three possible codons encode for the amino acids alanine, aspartic acid and glycine. The nucleotide sequence N-terminal tyrosine residue was changed in THT describing thymine (first position), adenine, cytosine or thymine at the second position and thymine (third position). These codons encode isoleucine, leucine and phenylalanine. Additionally, the sequence of phenylalanine in the core peptide sequence was shift to NNK. NKK stands for one of the four nucleotides at the first position, and guanine or thymine at the second and third position. Consequently, this codon encodes the amino acids arginine, glycine, tryptophan, methionine, leucine, valine, serine, cysteine, isoleucine and phenylalanine.
To construct this library a fill-in reaction using the designed forward and reverse oligonucleotides was performed. Subsequently resulting randomized oligonucleotide was digested with the restriction enzyme AatII. In addition, the vector pUP089 was digested with the restriction enzymes AatII and SfoI. After ligation of fragments, randomized oligonucleotide and vector, the focused library 2 (ligation product) was transformed in chemocompetent E. coli XL1-Blue cells. This procedure was done several times and a library size of 1233 colonies was reached.
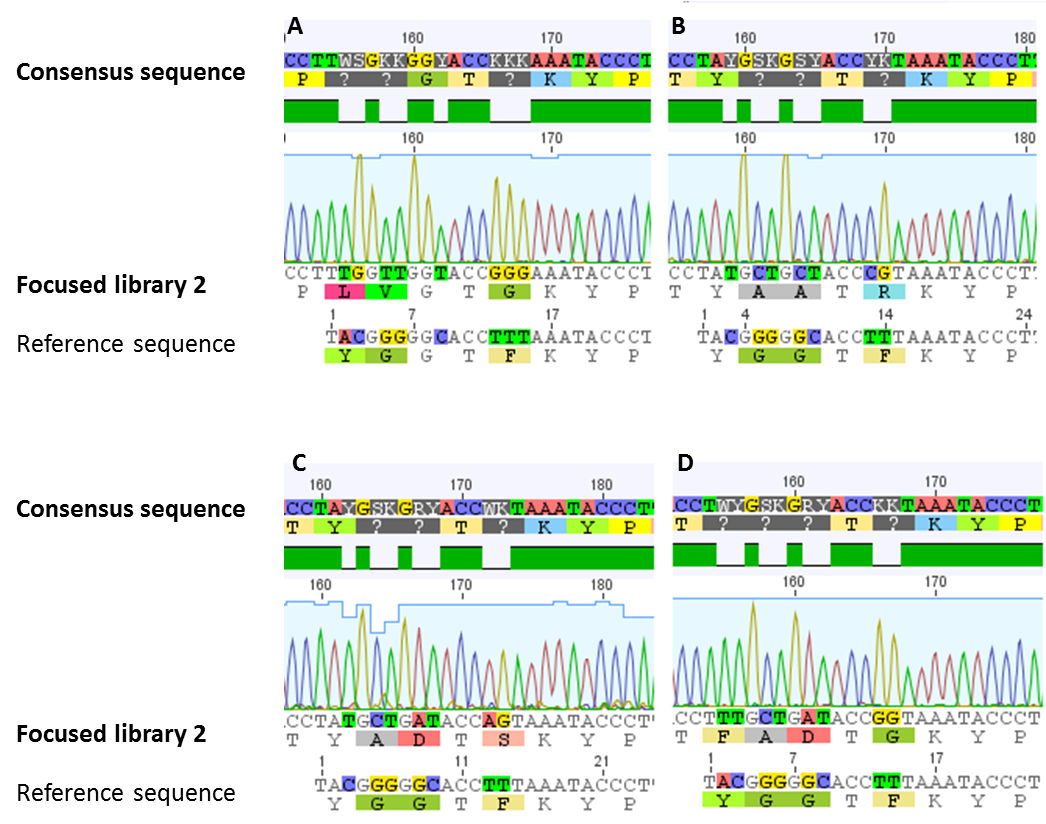
For confirmation of the focused library 2, sequencing analysis of a number of clones was performed. In all of our sequenced samples the requested modifications were established (figure 7). A sequence logo was generated for the 14 amino acid sized core peptide of MdnA. This sequence logo, which is illustrated in figure 8, indicates that the desired sites stay conserved. The others are modified.
The generated and modified focused library 2 should be used as basis for in vivo selection and phage display screening. Furthermore we purpose the objective to search for optimized and novel microviridins by using this library.
Expression Backbones
In a subtask of our work we wanted to construct auxiliary expression backbones with inducible promotors. In contrast to constitutive systems inducible systems only express the protein of interest after adding an inducer, which can be for example AHL or arabinose. We decided to construct IPTG- and arabinose-inducible systems. Therefore we amplified the promotor region and fused it to a reporter gene, which is constituted by YFP. Using this we have the option to verify the presence of the inducible promotor and also the function of the induction process by fluorescence. Our idea was to use the reporter gene as a placeholder. Via restriction enzyme digestion using the iGEM restriction enzyme sites you will be able to replace the reporter gene with your gene of interest. As vector backbone we made use of the pSB1A3, which has ampicillin resistance (Fig. 9). The constructs using pSB1K3 (kanamycin resistance) and pSB1C3 (chloramphenicol resistance) are still on the anvil.
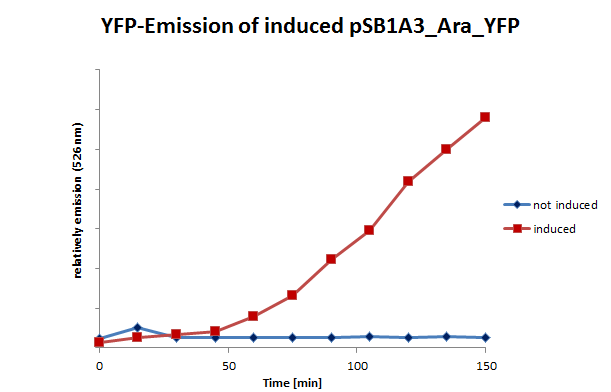
We successfully tested the IPTG- and arabinose-inducible system (Fig 10). Using fluorescence microscopy we were able to detect the YFP-expression after an induction time of approximately 1.5 hrs. To prove the hypothesis we opposed an induced sample with a non-induced control for each promotor. By use of brightfield combined with differential interference contrast microscopy we traced the E. coli cells and switched then to the YFP detecting channel to investigate the fluorescence of these cells.
We also tested the inducible systems by using fluorescence spectroscopy. For this experiment we induced both systems at a time when the cultures were located in phase of exponential growth. After inducing, the cultures were analyzed by fluorescence spectroscopy. In this analyze the cells were excited by 500 nm. The resultant emission was measured in a spectrum between 510 and 580 nm. (Fig 11;12)
The lacking of the LacI gene on the vector is the reason for expression of YFP in both controls. For further research in this field it is necessary to clone the LacI gene inside the constructed vector.
References
Ziemert, N., Ishida, K., Liaimer, A., Hertweck, C. & Dittmann, E. (2008). Ribosomal synthesis of tricyclic depsipeptides in bloom-forming cyanobacteria. Angewandte Chemie (International ed. in English) 47, 7756-9
Ziemert, N., Ishida, K., Weiz, A., Hertweck, C. & Dittmann, E. (2010). Exploiting the natural diversity of microviridin gene clusters for discovery of novel tricyclic depsipeptides. Applied and environmental microbiology 76, 3568-74
 "
"