Team:HokkaidoU Japan/Project/RFC87
From 2011.igem.org
HokkaidoU Japan
iGEM 2011 Team of Hokkaido University
Contents |
- Abstract
- What`s T3SSDetailed information about T3SS and summary of our achievements on iGEM 2010
- Injection assay using onion cellsExperiments using plant cells are easier to perform than with mammalian ones
- Ready-to-inject backbone and Bsa I cloning siteReady-to-inject backbone and Bsa I cloning site enables easy fusion of T3S signal and protein
- GSK tag systemA neat injection assay using GSK tag, which can specifically detect successfully injected proteins
- Bsa I cloning site, RFC submissionDetailed documentation of costructing a BioBrick cloning site a BioBrick!
Graphical explanation for Bsa I cloning site
Purpose
We designed "Bsa I cloning site" to accomplish following requiments.
- To produce a fusion protein.
- To insert parts universally.
- To produce a new BioBrick part itself.
- To insert parts only in correct direction.
It is ideal that the insert is cut with BioBrick official restriction enzymes (requirement 2) however, cloning site must be cut without using those enzymes (requirement 3), and each overhang must be different (requirement 4).
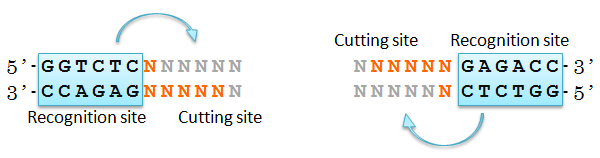
Restriction Enzyme Bsa I
We came up with an idea of using restriction enzyme Bsa I. Bsa I recognition site and its cutting site are separated, and the sequence of cutting site can be designed as you like.
Design
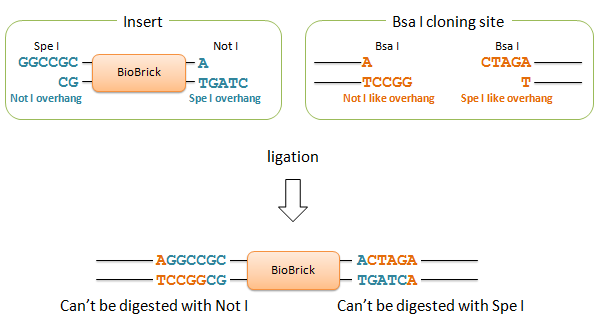
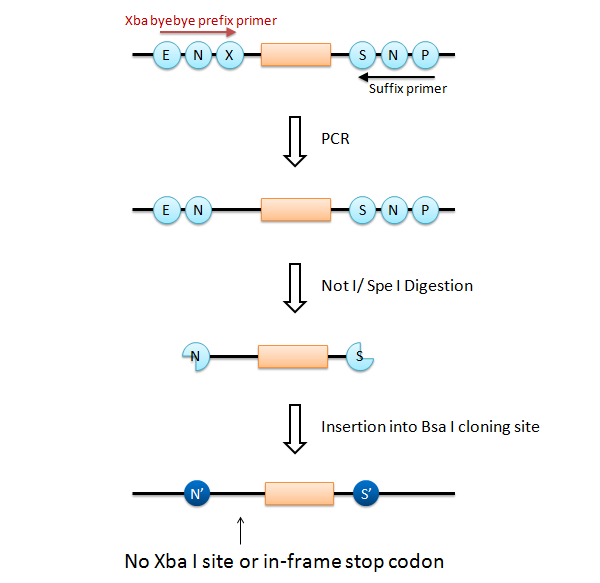
We designed two Bsa I site to produce Not I like overhang and Spe I like overhang respectively. These cutting ends are ligated with Not I/ Spe I digested BioBricks in correct direction, and the product cannot be re-digested by Not I or Spe I. We can amplify every insert part using Prefix and Suffix primer set.
Problems
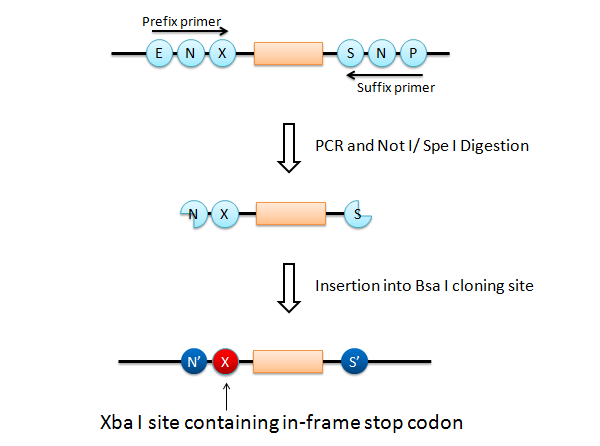
If this goes on, we have a serious problem that the final product has Xba I site after the Not I site (fail to fulfill the requirement 3). What is worse is that an in-frame stop codon appears on the Xba I site, just before the insert.
Solution?
To solve this problem, it is easy to add only a Not I site and a Spe I site at each end of the insert part. However, it requires different primer set that anneals each insert part (fail to fulfill the requirement 2).
Solution!
We designed a new universal prefix primer named "Xba_byebye". This primer causes a point mutation to delete the Xba I site and its internal in-frame stop codon. Using the "Xba_byebye" prefix primer and the normal suffix primer, we can fulfill all the requirements. After PCR, the problem template DNA can be removed by Dpn I digestion.
Application
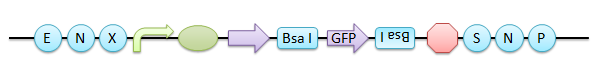
In this construct, the inserted GFP is interchangeable with another BioBrick part using Bsa I again, you can easily identify the replacement of the insert by the color of colonies.
We have submitted this method as BBF RFC 87.
 "
"