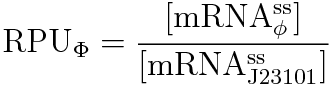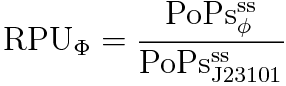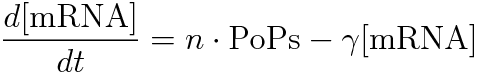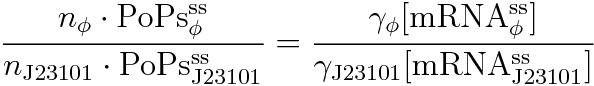Team:Kyoto/Hunger
From 2011.igem.org
Contents |
Project Hunger
Production of useless enzymes is a heavy burden especially when resources are scarce. This can be reduced by using nitrogen regulatory proteins, NtrB and NtrC† which activate the σ54promoter when the supply of nitrogen is insufficient. NtrB and NtrC are coded in glnL and glnG, respectively. However, the σ-54 promoter has never been evaluated quantitatively.
We characterize σ54promoter by Relative Promoter Unit (RPU), because absolute promoter activity depends on test conditions and measurement instruments. RPU can reduce this Coefficient of Variation (CV) from 39.1% to 17.5% [2]. Therefore RPU can make it easier for us to share the data of promoter activity and use BioBricks.
We have used GFP fluorescence to measure RPU, but this time, we tried to calculate RPU another way for this reason:
- We know that it is a lot of trouble to calculate RPU using GFP fluorescence.
- We imagined that the concentration of the glutamine changes because σ-54 promoter relates to the metabolism of glutamine.
This is because when we calculate RPU using GFP, we need measure the concentration of glutamine at two points in an exponential growth phase, but we have following problems.
- before an exponential growth phase, the concentration of glutamine changed.
- the concentration of glutamine is different between one point and the other point.
We devised the new way of calculating RPU using the amount of mRNA. In this way we use the following equation.
† NtrB and NtrC are otherwise called NRII, NRI
ところでこの遺伝子はもともとBioBrickに登録されていたものか、今回新たに作ったもののどちらなのでしょう
http://partsregistry.org/wiki/index.php/Part:BBa_J64978
などがもう登録されているようですが
リンク先を見ましたがどうなんでしょうね とりあえずこの登録されているパーツのシーケンスは見ましたが今回我々が作ろうとしたglnLとは全然違うシーケンスでした 名に由来かは知りませんがE.coliのものじゃないでしょうねby Hashiya
Introduction
For every living thing, needless biological activity is not efficient. Cells must be controlled so that enzymes are produced only when they are necessary. Ammonia is an essential nitrogen source for the bactria. When enteric bacteria are deprived of ammonia, they express glnA to produce glutamine synthetase(GS) under the &sigma54promoter. The transcription from &sigma54promoter is stimilated by phosphorylated form of NtrC(NtrC-P). The &sigma54 RNA polymerase binds to the glnA promoter, forming a closed complex, but cannot form an open complex and initiate transcription until it is activated by NtrC-P. NtrC is phosphorylated by NtrB-P, an autokinase which phosphorylates itself with ATPs.
Nitrogen is used in the reaction of
Glutamate + NH3 + ADP → Glutamine + ADP + phosphate
GS
The expression of glnA is regulated by several proteins including NtrB, NtrC, Pii.
Fig. NtrCがσ54,RNAポリメラーゼ,DNAの関係図(窒素源あり/なし) Fig. NtrBCの関係図 Fig. Ntr, Pii, GSの関係図
Method
We created two following constructions to measure gene expression depending on the concentration of glutamine. One construction includes BBa_J23101 as a promoter which is used as the standard and the other includes binding sites and σ-54 promoter.
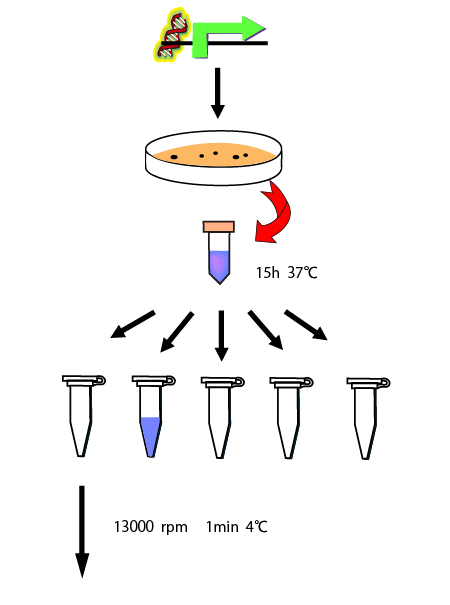
We also measured the amount of mRNA to calculate RPU of the steady state for each concentration of glutamine. First, we made following preliminary experiment to measure the length of times before steady state.
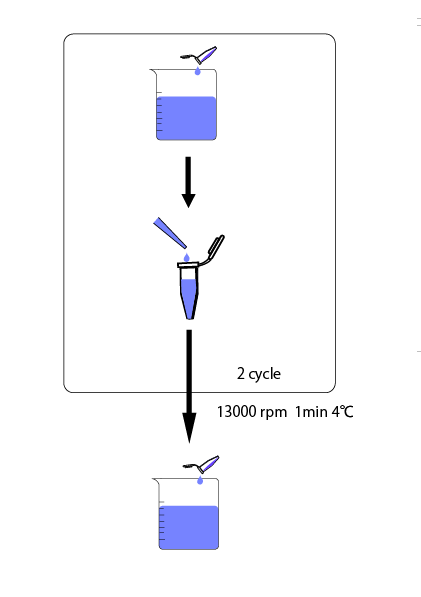
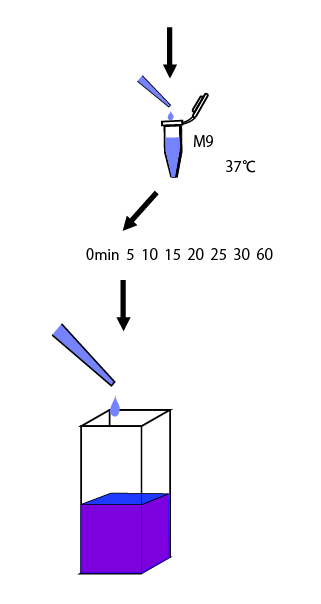
We cultivated E.coli in M9 media(+ casamino acids) for about 15 hours and dispenced 2.4ml to each tube. Then, we centrifuged these tubes (13,000 rpm , 4°C, 1min) and discarded the supernatant. We added 1.2ml media(- casamino acids) and centrifuged at 4°C twice. Again, we centrifuged these tubes and discarded the supernatant and added 1.2ml media(-casamino acids) at 37 °C. We brought up E.coli at 0,5,10,15,20,25,30,60min and extracted RNA and synthesized cDNA. Finally, we used real time PCR.
RPU is defined as follows.
This time, we decided to use the equation(2).
PoPS (Polymerase Per Second) is the unit of absolute “promoter activity”. It is defined as the number of RNA polymerase molecules that pass by the final base pair of the promoter and continue along DNA as an elongation complex. PoPSSS is PoPS at the steady state of following system.
Where:
- [mRNA] is the concentration of mRNA,
- γ is the mRNA degradation rate,
- n is the copy number of the plasmid containing the promoter
Following equations related to promoterφ and J23101 are derived because d[mRNA]/dt = 0.
If the test promoter φ and the reference standard promoter are measured under the same culture conditions and both promoters are carried on the same backbone plasmid, following equations are approved.
Equation(4) divided by (5) is (8) and we can reduce (8) because of (6) and (7).
The equation (2) is derived from the above.
So, we can calculate RPU this equation.
When we use this equation, we need following data:
- the amount of mRNA which is corrected by internal control
- when an expression level reach a steady state
This time we intend to assay a promoter activity against glutamine concentration, it is desirable to induct after cultivation in medium without glutamine. However, from our last year experience, we know that in M9 medium without casamino acids, E.coli will grow really inefficiently. So, at first we cultivated E.coli in the medium with casamino acids overnight, then changed the medium with the one without casamino acids. After that, we performed an experiment to determine how long they take to reach a steady state.
Result
Gragh1 The data, which is not corrected by internal control
- Although the amount of RNA is different first, we think more than the twice difference is not accidental error of the experiment.
- After 15 minutes, expression level greatly changed, but from 0 to 10 min, expression level is steady state.
- We think TBP and PGK are unsuitable for internal control.
- The behaviors of GAPDH and actin is analogous.
Gragh2~5
The data, which is corrected by internal control
- when we applied internal control to GAPDH, the changes of actin are little(×1.0~1.2)
- when we applied internal control to actin, the changes of actin are little(×0.8~1.0)
- We concluded that GAPDH and actin are suitable for internal control.
Discussion
Reference
Jiang, P., & Ninfa, A. J. (2007). Escherichia coli PII signal transduction protein controlling nitrogen assimilation acts as a sensor of adenylate energy charge in vitro. Biochemistry, 46(45), 12979-96. doi:10.1021/bi701062t
Kelly, J. R., Rubin, A. J., Davis, J. H., Ajo-franklin, C. M., Cumbers, J., Czar, M. J., Mora, K. D., et al. (n.d.). reference standard. Bioinformatics. doi:10.1186/1754-1611-3-4
Al-Azri, M., Al-Azri, H., Al-Hashmi, F., Al-Rasbi, S., El-Shafie, K., & Al-Maniri, A. (2011, May). Factors Affecting the Quality of Diabetic Care in Primary Care Settings in Oman: A qualitative study on patients’ perspectives. Sultan Qaboos University medical journal. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21969892
Reitzer, L. J., & Magasanik, B. (1986). Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell, 45(6), 785-92. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2871943
Magasanik, B. (1989). Regulation of transcription of the glnALG operon of Escherichia coli by protein phosphorylation. Biochimie, 71(9-10), 1005-12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2574599
Keener, J., & Kustu, S. (1988). Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proceedings of the National Academy of Sciences of the United States of America, 85(14), 4976-80. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=281670&tool=pmcentrez&rendertype=abstract
Two-component and phosphorelay systems Signal transduction systems. (n.d.).http://www2.hawaii.edu/~scallaha/SMCsite/475%20Lectures/475Lecture36.pdf
 "
"