Team:TzuChiU Formosa/Notebook/photopaper
From 2011.igem.org


Photopaper
2011.02.24
Discussion:
- Team organization
- Brain storming
- paper made by bacteria with add-ons such as colors, fragrance, etc.
- "light up" the plants for replacing lamp posts.
2011.03.04
Discussion:
- Team advisory
- Brain storming
- Add-ons: colorful cellulose which produced by bacteria such as beta-carotene
- information exchange with iGEM 2009 Cambridge team
2011.03.14
Discussion:
- Task Allocation
- Brain storming
- Avatar - "light up" the plants by transfecting symbiotic bacteria which cloned into fluorescence gene
- Eco-friendly warmer - biotic thermal pad
2011.03.23
Discussion:
- Project : paperia
- Option 1 : Culture bacteria which has pigment gene
- Option 2 : Cellulose-producing bacteria secrete pigment into the medium
2011.03.24
Discussion:
- Exp. procedure:
- cloning of cellulose gene’s CDS
- the product should operate within E. coli.
2011.06.22
Discussion:
- Due to some unforseen reason, the team decided to change their project.
- New project: Biojenny
- -economical and humane way to produce paper in large quantities.
- -yeast to be our host
2011.07.01
Discussion:
- Freeze > grin > genome DNA isolation > Cloning = silk protein gene
2011.07.09
Discussion:
- the connections between 3 silk proteins : Fibl Fibh P25
- major proteins : H-chain, L-chain, P25
2011.07.15
Discussion:
- Due to limited resources and techniques required, the team decided to switch back to the previous project. Paper making !
- However it would be modified to be more innovative and creative.
2011.07.18
Discussion:
- Latest project : Photo paper
- cyanobacteria is designed as the host, cellulose made up of the glucose produced by cyanobacteria could be one of the main attraction of the project.
2011.07.23
Discussion:
- system modification to overcome the problems arises during preliminary round
- Biobricks from Tokyo 2010 team will be utilized
- regulator promoter in order to regulate the secretion of cellulose to solve the aggregation of the bacteria
2011.09.07
'''Rhodobacter rudrum medium'''
K2HPO4 1g
NaCl 0.5g
FeSO4.7H2O 0.01g
CaCl2 0.02g
MnCl2.4H2O 0.002g
MgSO4.7H2O 0.2g
NaMO2O4.2H2O 0.01g
ddH2O 998.258 ml
(+
______________________________________________________
1L→take100ml
+
Yeast Extrat 0.5g
Sodium malate
(Sodium succinate dibasic hexohydrate) 5g
NH4Cl 1g
ddH2O 893.5ml
(+
______________________________________________________
1L
'''Raise E. coli(PSB1C3)'''
1.50ml LB+500μl CHLORAMPHENICOL
2.37℃, overnight (14-16hrs)
2011.09.08-13
Plasmid miniprep kit PSB1C3 plasmid
Raise Rhodobacter rubrum
1.50ml LB+500μl CHLORAMPHENICOL 2.37℃, overnight (14-16hrs)
2011.09.09
Raise Gluconacetobacter hansenii 1.50ml LB+500μl CHLORAMPHENICOL 2.37℃, overnight (14-16hrs)
2011.09.10-11
'''Digestion check of DNA'''
PSB1C3 DNA (control) 3μl
PSB1C3/ EcoR
DNA 500ng
10×buffer 5μl
BSA 5μl
EcoRⅠ 1μl
ddH2O 29μl
(+
______________________________________________________
50μl
PSB1C3/ pstⅠ
DNA 500ng
10×buffer 5μl
BSA 5μl
pstⅠ 1μl
ddH2O 29μl
(+
______________________________________________________
50μl
PSB1C3/ EcoRⅠ+pstⅠ
DNA 500ng
10×buffer 5μl
BSA 5μl
EcoRⅠ 1μl
pstⅠ 1μl
ddH2O 28μl
(+
______________________________________________________
50μl
→37℃ for 30 mins
'''Digestion of DNA'''
PSB1C3/ EcoRⅠ+pstⅠ
DNA 10μl
10×buffer 5μl
BSA 5μl
EcoRⅠ 1μl
pstⅠ 1μl
ddH2O 28μl
(+
______________________________________________________
50μl
→37℃ for 2 hrs
'''electroelution Purification'''
PSB1C3 backbone
2011.09.14-24
PCR
template DNA 1μl
5×Buffer 4μl
2.5μM dNTP 1.6μl
10μM F 1μl
10μM R 1μl
Taq 0.2μl
ddH2O 8.8μl
_______________________________
total 20μl
2011.09.15
'''Genome miniprep''' Gluconacetobacter hansenii
2011.09.18
'''Gel/PCR DNA extraction''' Gluconacetobacter hansenii
2011.09.21
'''Digestion of DNA'''
acsAB/ XbaⅠ+SpeⅠ
DNA 10μl
10×buffer 5μl
BSA 5μl
EcoRⅠ 1μl
pstⅠ 1μl
ddH2O 28μl
(+
______________________________________________________
50μl
→37℃ for 16 hr
'''Digestion of DNA'''
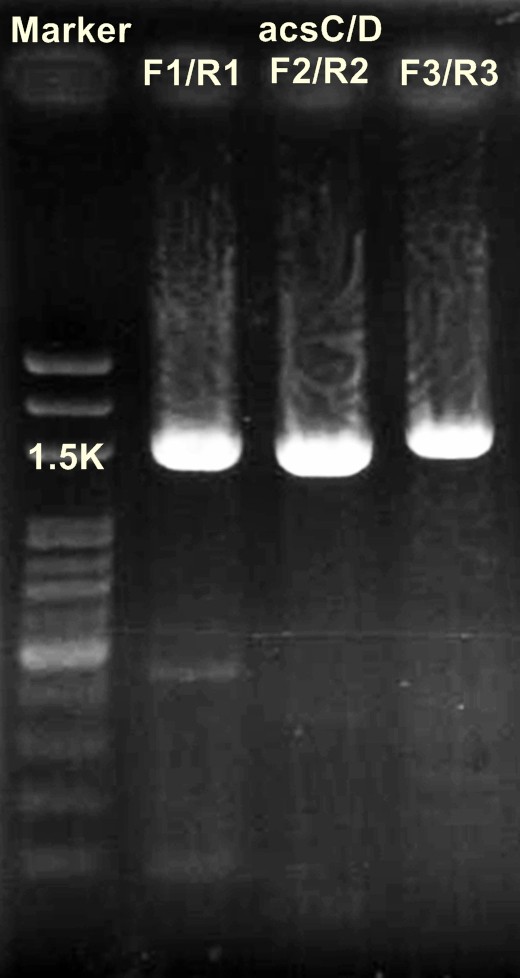
acsCD/ XbaⅠ+SpeⅠ
DNA 10μl
10×buffer 5μl
BSA 5μl
EcoRⅠ 1μl
pstⅠ 1μl
ddH2O 28μl
(+
______________________________________________________
50μl
→37℃ for 16 hr
2011.09.22
'''Ligation of DNA'''
PSB1C3-acsAB
Vector 3μl
Insert 14μl
ligase buffer 2μl
ligase 1μl
ddH2O -μl
(+
______________________________________________________
20μl
'''Ligation of DNA'''
PSB1A3-acsCD
Vector 3μl
Insert 14μl
ligase buffer 2μl
ligase 1μl
ddH2O -μl
(+
______________________________________________________
20μl
2011.09.23
'''PCR'''
PR0011 promoter 1μl
5×Buffer 4μl
2.5μM dNTP 1.6μl
Taq 0.2μl
ddH2O 13.2μl
(+
______________________________________________________
20μl
2011.09.24
'''Transformation of DNA''' PSB1C3-acsAB Transform into E.coli LB+CHLORAMPHENICOL '''Transformation of DNA''' PSB1A3-acsCD Transform into E.coli LB+Ampicillin '''Transformation of DNA''' PSB1C3-acsAB PSB1A3-acsCD Transform into E.coli LB+Ampicillin+CHLORAMPHENICOL
2011.09.24
'''Ligation of DNA'''
PSB1C3-promoter
Vector 3μl
Insert 14μl
ligase buffer 2μl
ligase 1μl
ddH2O -μl
(+
______________________________________________________
20μl
 "
"