Team:Waterloo/Notebook
From 2011.igem.org
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | File:Waterloo logo.png 200px |
|
Tell us more about your project. Give us background. Use this is the abstract of your project. Be descriptive but concise (1-2 paragraphs) | File:Waterloo team.png Your team picture |
| Team Example |
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Notebook
You should make use of the calendar feature on the wiki and start a lab notebook. This may be looked at by the judges to see how your work progressed throughout the summer. It is a very useful organizational tool as well.
The following entries pertain to the Quantification Project
Tuesday, May 31, 2011
- Transformation of BBa_I20260 from iGem Kit Plate 2, Well 17F.
Wednesday, June 1, 2011
- Created a frozen stock of I20260
- Inoculated BBa_I0500 and BBa_E0240 from frozen stock already made from last year
Thursday, June 2, 2011
- Miniprepped and nanodropped I0500, and E0240.
- Inoculated I20260 from the frozen stock created the previous day
Friday, June 3, 2011
- Digestion Reaction. Digested I0500 with EcoRI and SpeI. Digested E0240 (first sample) with EcoRI and PstI and digested E0240 (second sample) with EcoRI and XbaI.
- Miniprepped and nanodropped I20260
Monday, June 6, 2011
- Inoculated I20260, I0500 and E0240
Tuesday, June 7, 2011
- Miniprepped and nandodropped I20260, and I0500 and E0240 for extra sample
- Inoculated E0240
Wednesday June 8, 2011
- Digestion reaction. Digested I0500 with EcoRI and SpeI. Digested E0240 (first sample) with EcoRI and PstI and digested E0240 (second sample) with EcoRI and XbaI. Digested I20260 with EcoRI and PstI.
- Miniprepped and nanodropped E0240 for back up
Thursday June 9, 2011
- Gel extraction of E0240 (EcoRI+XbaI), I0500 (EcoRI+SpeI), E0240 (EcoRI+PstI) and I20260 (EcoRI+PstI). Two samples of each parts were gel extracted.
- Nanodropped the samples from Gel extraction. Concentration of I0500 and I20260 were too low.
- Inoculated I0500 and I20260
Friday June 10, 2011
- Miniprepped I0500 and I20260 and ran digestion reaction. Same enzymes were used as the ones listed above.
Tuesday, June 14, 2011
- Gel extraction of I0500, I20260 and the back-up samples of E0240.
Thursday, June 16, 2011
- Ligation reaction: Ligated I0500 on to E0240. Ligated I20260 on to pSB1A2 (from E0240)
Monday, June 20, 2011
- Transformation of ligated parts (I0500+E0240, and I20260 on pSB1A2). Plated the transformant and incubated overnight
Tuesday, June 21, 2011
- Created plates containing 1% arabinose.
- Replicated plates containing I20260 colonies on to a different plate with Ampicilin antibiotic. Incubated overnight.
Wednesday, June 22, 2011
- Replicated I0500+E0240 to plates containing 1% arabinose. Incubated overnight
- Inoculated a single colony from I20260 plate
Thursday, June 23, 2011
- Created frozen stock of I20260
- Inoculated a single colony from I0500+E0240 plate
Friday, June 24, 2011
- Created frozen stock of I0500+E0240
Monday, July 4, 2011
- Inoculated the I20260, and I0500+E0240
- Created 1M Arabinose solution
Tuesday, July 5, 2011
- Made serial dilutions of arabinose solution (from 1M to 1uM).
- Diluted the cultures to 0.50 Absorbance at OD600.
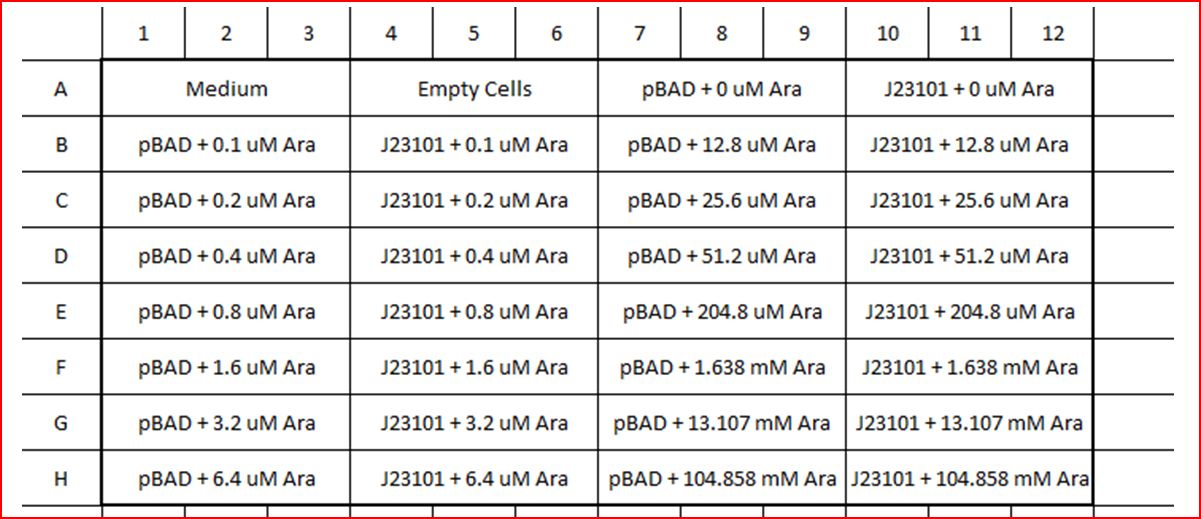
- Set up experiment for quantification experiment. Refer to the diagram below for how the experiment was setup.
- Monday, July 11, 2011
- Inoculated the I20260, and I0500+E0240
- Created 1M Arabinose solution
Tuesday, July 12, 2011
- Another quantification experiment run. Machine failure.
Wednesday, July 13, 2011
- Inoculated the I20260, and I0500+E0240
- Created 1M Arabinose solution
Thursday, July 14, 2011
- Last quantification experiment run.
The following entries pertain to the Ribozyme Project
Wednesday July 6, 2011
- Received 3 of 4 sequences the previous week (IN1, IN2 and GFP2).
- A quick spin down UWAT014-3/UWAT014-2 using centrifuge.
- Resuspended DNA in 40ul of MQ water (Concentration: 2ug/40ul=1ug/20ul)
- Transformed into DH5-alpha (sequences in PUC57).
- grown overnight on ampicillin plates.
- Resuspension of PSB1C3 in the Spring 2011 distribution kit (Plate 1 well 3A). Contains BBa_J04450.
- Resuspension of PSB1C3 in 10ul of MQ water (aspirated), wait approximately 5 minutes.
- 1ul of resuspension was transformed into DH5-alpha. Grown overnight on plate.
Thursday July 7, 2011
- IN1 (amp), IN2 (amp), GFP2 (amp) and PSB1C3 (cm) broth cultures innoculated (3 each).
Friday July 8, 2011
- Frozen stock of IN1, IN2 and GFP2 in PUC57 and PSB1C3 backbone made.
- Miniprep for IN1, IN2, GFP2 and PUC57 completed:
| Sequences | IN1 | IN2 | GFP2 | PSB1C3 |
| 260/280 | 1.85 | 1.80 | 1.88 | 1.86 |
| ng/ul | 229.8 | 236.1 | 198.6 | 166.2 |
- GFP1 Sequence(588nt)in PUC57 received from Bio Basic Canada INC.
Tuesday July 12, 2011
- Liquid cultures of GFP1 (x2), IN1, IN2, GFP2 and PSB1C3 were innoculated with the appropriate antibiotic in the broth.
Wednesday July 13, 2011
- GFP1, IN1, IN2, GFP2 and PSB1C3 were minipreped to isolate plasmid DNA.
- Frozen stock of GFP1 made.
Thursday July 14, 2011
- GFP1, IN1, IN2, GFP2, PSB1C3 digested with EcoRI and PstI. GFP2 also digested with ndeI.
- Innoculation of liquid culture (GFP1, IN1, IN2, GFP2, PSB1C3).
Fridat July 15, 2011
- Gel extraction of GFP1, IN1, IN2, GFP2 and PSB1C3. However, the results were not as anticipated.
- Miniprep of cultures innoculated yesterday.
Monday July 18, 2011
- Cultures were miniprepped, however, GFP1 did not have a sufficient concentration to undergo digestion.
- Proceeded with digestion for GFP2 (ndeI), IN1, IN2 and PSBIC3 with EcoRI and PstI.
- Innoculation of GFP1 (x4)
Tuesday July 19, 2011
- Miniprep and digestion of GFP1.
- Gel extraction of each digestion (PSB1C3(x2), GFP1, IN1, IN2, GFP2).
Wednesday July 20, 2011
- Lox resuspended and digested.
- Ligation of GFP2, IN1, IN2 and Lox into PSB1C3.
Thursday July 21, 2011
- Transformation of GFP2, IN1, IN2 and Lox. Each was plated on cm containing media and grown overnight.
Friday July 22, 2011
- All negative plates did not produce colonies.
- Growth was good on all positive plates except for IN1, which only produced two main colonies.
Monday July 25, 2011
- Gel extraction IN1, IN2 and GFP1, however, GFP1 failed.
- Ligation of IN1 and IN2 into PSB1C3.
Tuesday July 26, 2011
- Innoculation of GFP2 into cm containing LB broth tube and lox into amp containing LB broth tube.
- Gel extraction did not work (likely a problem with digestion or transformation)
- Innoculation x5 of GFP1.
Wednesday July 27, 2011
- Frozen stock of GFP2 and lox made
- Miniprep of GFP1 x4 replicates
- Gel extraction of IN1 and IN2 resulted in improved concentrations
- Transformation
July 30, 2011
- Miniprepped GFP 1 and GFP 2
August 2, 2011
- Miniprepped Intron 1 and intron 2.
August 3, 2011
- Digestion of GFP 1 and Intron 2 with SacI and EarI
- Digestion of GFP2 and Intron 1 with SacI and SapI
August 4, 2011
- Nothing
- Figuring out unexpected SacI cute site in the middle of pSB1C3 vector
August 5, 2011
- Nothing
August 6, 2011
- Digestion of GFP 1 with Bgl II and Ear I
- Digestion of Intron 1 with Bgl II and Ear I
- Digestion of GFP 1 with Ear I and Pst I
- Digestion of GFP 2 with EarI and Pst I.
- Gel extraction of all the samples listed above
August 7, 2011
- Ligation of GFP 1 with Intrton 1 and GFP 1 with GFP 2
- Transformation of the two ligation mixtures listed above
August 8, 2011
- Minipreping Int2, lox and GFP2
- Digestion
- Gel extraction
August 9, 2011
- Miniprepping BBa_K576007
- Digestion
- gel extraction
August 10, 2011
- Ligation and transformation of BBa_K576007 and J61046 to create K576009
August 11, 2011
- Ligation and transformation
August 12, 2011
- Streak plating GFP1-GFP2 (BBa_K576013)
- Backbone for RFC arrived and was streaked on cm plate
 "
"