Precipitator
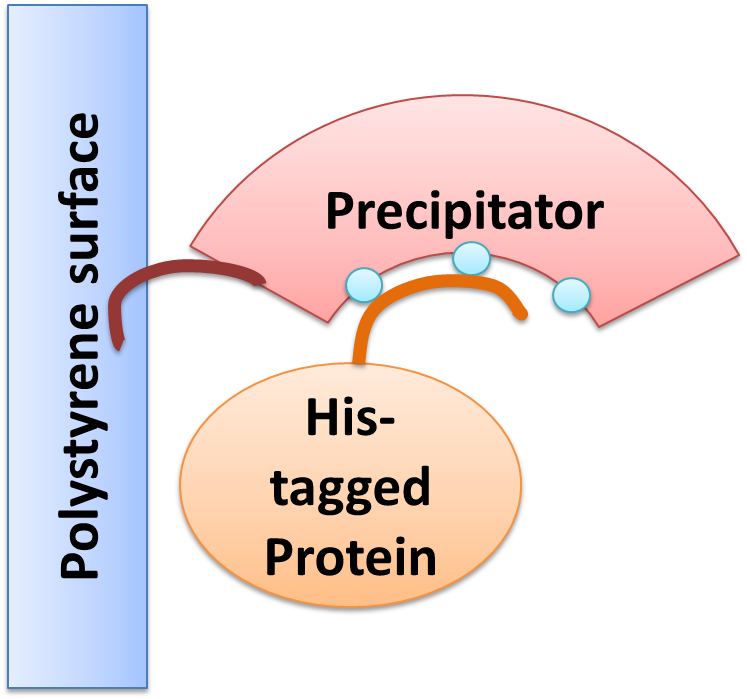
Precipitator binding polystyrene surface with the plastic binding domain and a His-tagged Protein via Nickel
We created a cellular, self-replicating purification device for His-tagged proteins. It is a completely artificial fusion protein, which consists of a repeating LRRNT motif domain, coordinating Ni2+ Ions on its surface capped on N and C terminal ends by a hagfish sequence of a similar LRRNT motif. A second domain, a short hydrophobic peptide stretch, binds a polystyrene surface. We named this construct “THE PRECIPITATOR”.
After expression of the Precipitator in a light inducible E. coli strain, the cells are lysed and the lysate is taken up by a serological pipette, in preparation of the actual protein purification steps.
The underlying mechanism is comparable to Ni-NTA columns. Our Precipitator protein binds on the surface of the pipette, presenting the chelated Nickel ions. Free coordination sites of the Nickel ions are exposed, so that a His-tagged protein can attach to them. Cells expressing a His-tagged protein can be dissolved by the heat inducible lysis device. Subsequently, when the lysate is taken up with a serological pipette coated with the Precipitator protein, the His-tagged proteins bind to it. Cell debris is then washed off, while the His-tagged protein stays and is eluted afterwards, in the same fashion as done in Ni-NTA columns with imidazole solutions, increasing in concentration. The His-tagged protein is finally captured in a distinct fraction.
Green light receptor
Blue light receptor
Red light receptor
Lysis cassette