Team:SJTU-BioX-Shanghai/Project/Subproject1/Design
From 2011.igem.org
|
|
DesignWe design a Rare-Codon Switch controlling protein biosynthesis. We can control the translation process by controlling three elements: rare tRNA amount aaRS rare codon
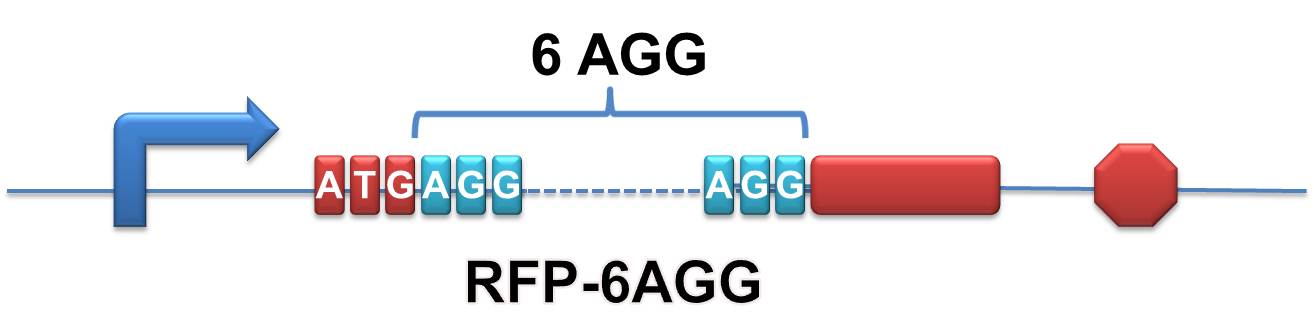
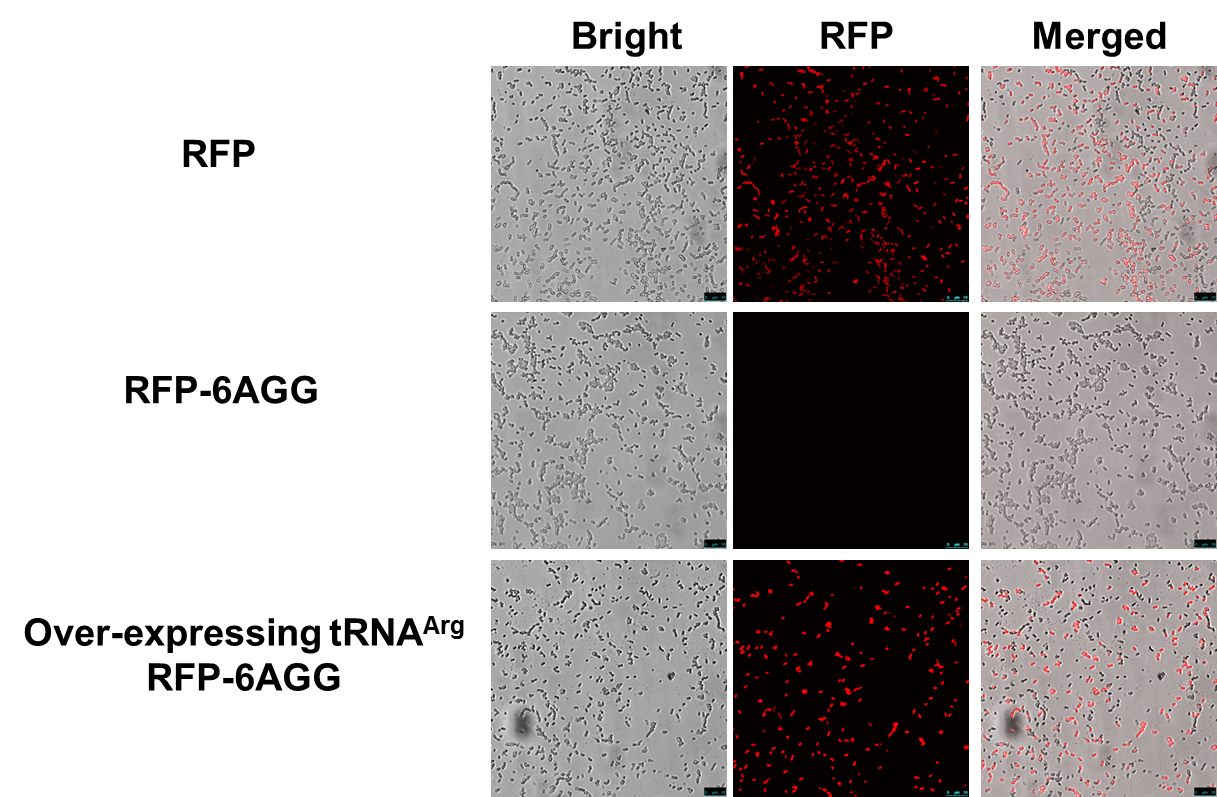
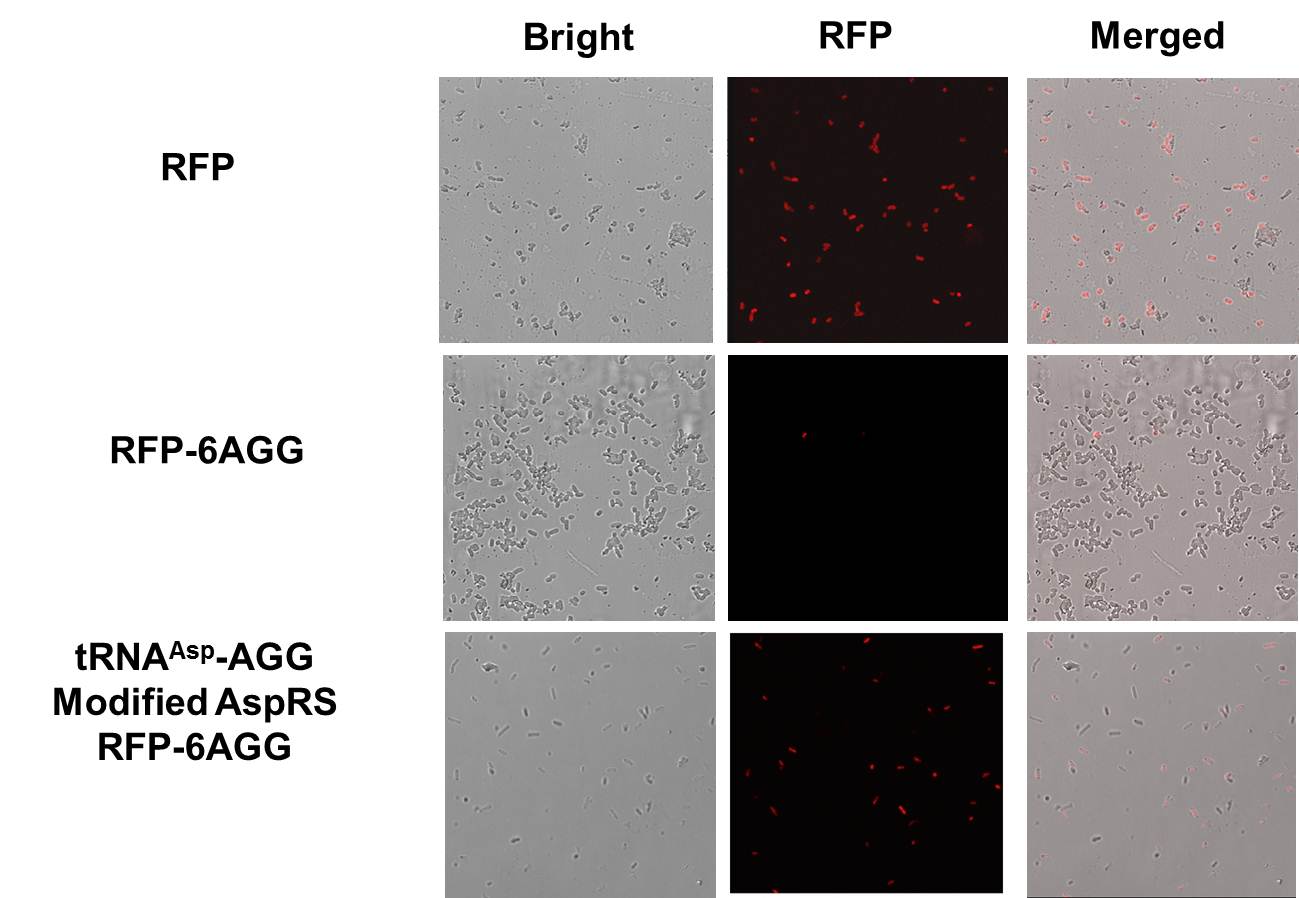
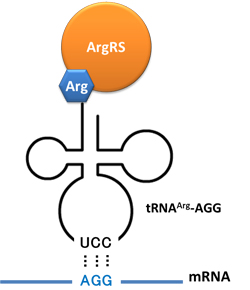
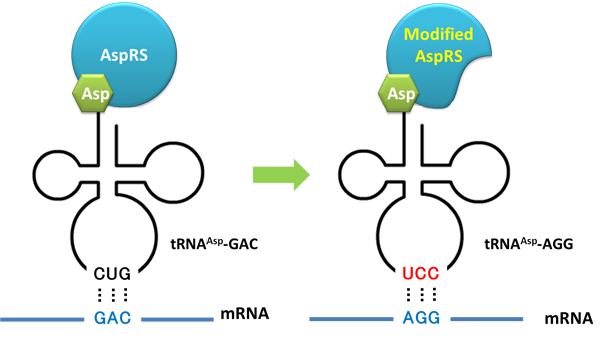
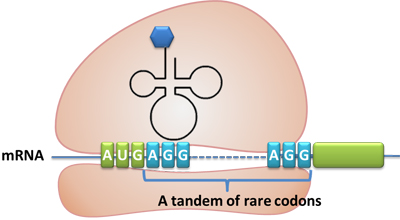
Rare tRNA AmountDesignIn this part we have overexpressed rare tRNAArg-AGG in the cell. The rare tRNA can recognize AGG codon on the mRNA. tRNAArg-AGG: tRNAArg-AGG is over expressed under the control of trc promoter (induced by IPTG). This rare tRNAArg can be charged with Arg by native Arginyl-tRNA Synthetase(ArgRS) in E.coli. 放图 RFP-6AGG: we have inserted 6 AGG codons after the start codon ATG in the RFP gene. ActionWhen rare tRNAArg-AGG is not over-expressed, RFP expression is hindered. When tRNAArg-AGG is over-expressed, this tRNA can recognize the AGG codon on the mRNA so a large amount of RFP is produced. ResultRFP has been largely produced in cells overexpressing tRNAArg. No RFP can be observed in cells without rare tRNA overexpression. We have successfully controlled protein expression by controlling rare tRNA amount. aaRSDesignWe control the translation process by modifying the tRNA and aaRS that are originally not for Arg. tRNAAsp-AGG: tRNAAsp with its anticodon mutated to CCU can base pair with rare codon AGG, which is originally the codon for Arg. This tRNA is under the constitutive aspV promoter. TDRS: Aspartyl aminoacyl tRNA synthetase (AspRS) without anticodon recognition domain under the control of T7 promoter and lac operator. To deprive AspRS of its anticodon specificity, we analyzed the structure of AspRS and expressed a truncated AspRS without anticodon recognition domain. This modified enzyme keeps its ability of aminoacylation while loses its activity of recognizing anticodon of tRNA. Reporter:We test our design with two reporters. RFP-6AGG: RFP with 6AGG insertions Luciferase-4AGG: luciferase with 4AGG insertions ActionWhen the modified enzyme is produced under induction, it can charge tRNAAsp-AGG with Arg. Then the charged tRNA can get through the rare codons on the mRNA, so that RFP or luciferase can be produced. ResultWe have used PT7-RFP-6AGG (BBa_K567017) as our Reporter. We have constructed tRNAAsp-AGG and PT7-TDRS (AspRS without anticodon recognition domain) (BBa_K567012 and BBa_K567011). tRNAAsp-AGG, which can recognize rare codon AGG, is under constitutive promoter. With our device, RFP is successfully produced. Without our device, little RFP is observed. 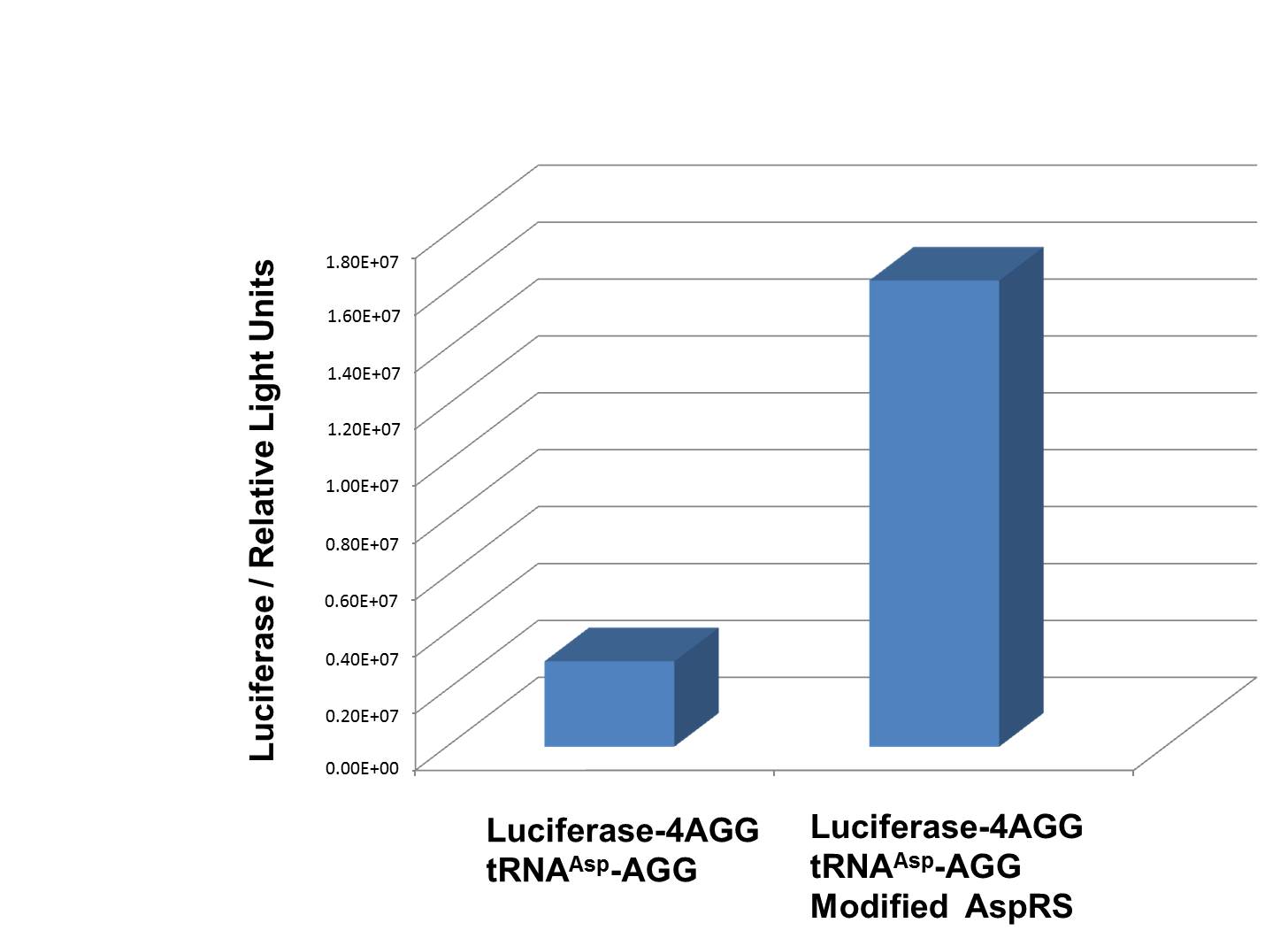 Fig 1. Examination of luciferase production with and without device. ER2566 cannot produce luciferase with PT7-Luc-4AGG (BBa_K567009) only. When tRNAAsp-AGG (BBa_K567012) and PT7-TDRS (BBa_K567011) are co-transformed into the cell, luciferase production is increased. The results proved that aaRS can regulate protein biosynthesis. We have used PT7-Luc-4AGG (BBa_K567009) as our Reporter to test the function of PT7-TDRS (BBa_K567011) and tRNAAsp-AGG (BBa_K567012). Results are shown above. Luciferase production has been largely increased with our device. The function of Switch is characterized by the amount of luciferase expressed. The amount of luciferase expressed is reflected by the light emitted when luciferase acts on the appropriate luciferin substrate. The light can be measured by luminometer and the quantity is positively correlated with the amount of luciferase and its activity (learn more...). We successfully controlled protein expression by manipulating aaRS. Number of Rare CodonsIn this part we want to explore the influence of the number of rare codons inserted in the mRNA. We have inserted 2, 4, 6, 8 AGG codons respectively after the start codon in luciferase gene. T7 promoter or bla promoter[1] are used to control target protein mRNA amount. We use different combinations of number of AGG codons and strength of promoters to characterize regulation[1]. 1) bla promoter-luciferase (weaker promoter) A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase 2) T7 promoter-luciferase (stronger promoter) A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase Influence of inserted AGG codon numberThe influence of different number of rare codons in regulating protein biosynthesis is shown below: 贴图 T7 BLA 2 4 6 8 Results show that the more rare codons are inserted, the lower the background expression and the narrower the range our device can regulate. 贴图registry fig 7 This picture reflects more clearly that the more rare codons are inserted, the lower the background expression and the narrower the range of device regulation. We are able to predict the outcome of influence of different number of rare codons in protein biosynthesis, offering valuable information for device usage. 贴图registry fig 11 Influence of different strengths of target protein promotersWe examined the influence of different Reporter promoters on the working curve of our device, which is reflected by luciferase activity. The working range of our device is pre-defined by the strength of target protein promoter, T7 promoter and bla promoter in our project. 贴图registry fig 8 Note: Our device can be used as a regulating toolWe have tested luciferin reaction in cells. We examined the changes in luciferase enzyme activity over time after rare tRNA expression is induced. The amount of luciferase is reflected indirectly by the bioluminescence emitted from the luciferin reaction. Results are shown below: 贴图 原来的图 看registry Here we use the above two curves as examples to characterize the working curve of our device. Both curves fits typical titration curve, indicating that our device can function as a regulating tool. The rest of the working curves are shown here: 贴图 原来的图 看registry Results showed that all the devices’ working curves fit titration curve, indicating that our device can act as a satisfying regulating tool. From this experiment, we noticed that the typical working curve of our device can be better observed under IPTG induced lacI-Ptrc-tRNAArg (BBa_K567001) compared with UV excitation induced sulA promoter-tRNAArg(BBa_K567002), though sulA promoter-tRNAArg responded quicker to signals. So in the above experiments, we test with lacI-Ptrc-tRNAArg. Location of Rare CodonsModulator
Click here to see Modeling. ReporterReporter controls the number of rare codons in the target protein's mRNA. We chose the rarest codon AGG for Arg in E.coli as our controlling element. A tandem of AGG codons is inserted after the ATG codon of reporter gene. 1. Reporter for Qualitative Analysis: A tandem of 6 AGG codons is inserted after the ATG codon of reporter gene RFP and GFP respectively. The fluorescence emitted reflects how well the ribosome can get through a tandem of rare codons in the target protein's mRNA, thus reflecting how well our system works. 2. Reporter for Quantitative analysis: We use luciferase as our reporter gene for quantitative analysis. The amount of luciferase expressed is reflected by the light emitted when luciferase acts on the appropriate luciferin substrate. The light can be measured by luminometer and the quantity is positively correlated with the amount of luciferase and its activity (Learn more...). We use different combinations of number of AGG codons and strength of promoters to characterize regulation[1]. 1) bla promoter-luciferase (weaker promoter) A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase 2) T7 promoter-luciferase (stronger promoter) A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase Click here to see Modeling. Action: Combinations of Modulators and ReportersSelect a Modulator and a Reporter, then click
Reference[1]Ulrich Deuschlel., et al., Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures The EMBO Journal vol.5 no. 11 pp.2987-2994, 1986 |
 "
"