|
|
Project
Overview
Bacteria protect their genome and remove foreign DNA elements through the use of the clustered, regularly interspaced short palindromic repeat (CRIPSR) system. Recently discovered, this defense mechanism can protect microorganisms against bacteriaphages. The CRISPR system comprises a transcribed locus of repeats and spacers that is activated and made functional via a family of CAS proteins. The CRISPR repeats are short DNA sequences that are almost identical in size and sequence. Between a pair of repeats is a spacer, which if complementary to a DNA element of the invading bacteriaphage, facilitates the destruction of that sequence from the bacterial cell. As such, the CRISPR/CAS system is a primitive biological defense mechanism, similar in many ways to the process of adaptive immunity in eukaryotic immune systems.
Plasmids, which are commonly used in molecular biology are also the source of horizontal gene transfer in the wild and facilitate the spread of antibiotic resistant genes among microorganisms. These plasmids can contain unique DNA sequences that could be targeted by the CRISPR/CAS system. We exploit the CRISPR/CAS system to engineer a mechanism of plasmid curing and deactivation of antibiotic resistance genes in E. coli.
We synthesized a version of CRISPR encoding a spacer that matches the GFP DNA sequence. We tested the synthetic CRISPR array against E.coli harboring a tetO::GFP plasmid that confers ampicillin resistance. Activation of CRISPER-GFP destroys the GFP-containing plasmid restoring the bacterial host's sensitivity to ampicillin. We will use the synthetic CRISPR system as a biological tool, combining it with other BioBricks for use in applications that will impact health and medicine, biotechnology, molecular biology, and genetics.
Details
Studies have shown that Escherichia coli bacteria have a system that provides immunity against viral infections and plasmid conjugation in the natural world. This recently discovered system, also known as the CRISPR/Cas system, is made of eight cas (CRISPR-associated) genes and an array of repeats and spacers known as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats). The repeats are repeating DNA sequences of approximately thirty nucleotides that are separated by unique genetic elements, spacers. The sequences of these spacers are generally derived from foreign virus DNA or plasmids. The CRISPR array expands when the bacteria collects a new spacer in a process known as CRISPR adaptation. The bacteria only confers resistance to the virus or plasmid when its CRISPR repertoire contains a spacer that matches the DNA sequence of the invading DNA. Although parts of the pathway for CRISPR adaptation and CRISPR interference are being studied, many mechanisms such as spacer integration into the CRISPR array as well as the degradation signaling pathway for CRISPR-targeted DNA remain to be discovered. Below is a schematic on a possible pathway the CRISPR/Cas system in E. coli utilize for CRISPR interference:
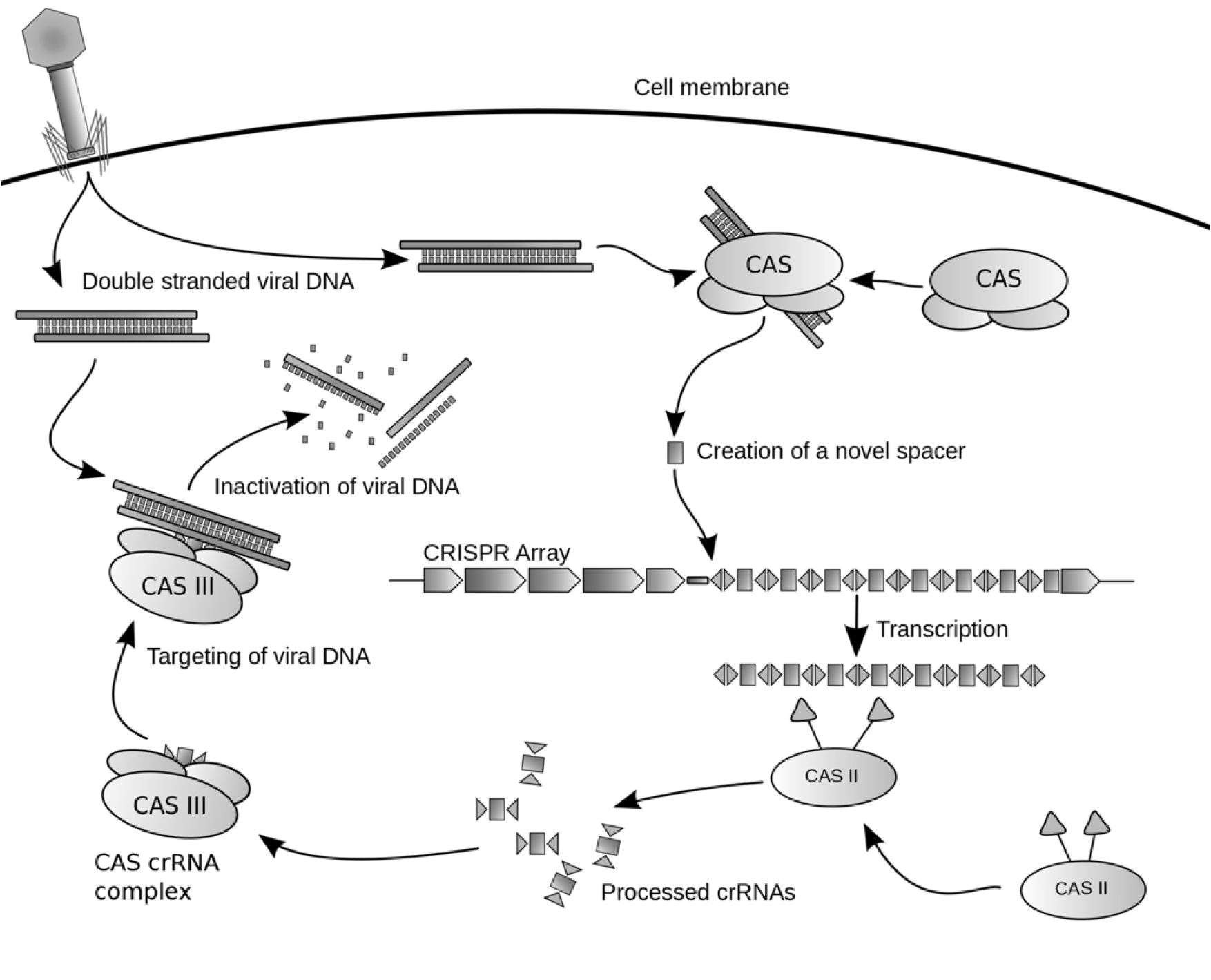 Possible mechanism of CRISPR/cas system against viral DNA Genetic Arrangement of CRISPR/Cas System
To the right is a schematic diagram of how the CRISPR/Cas system is arranged genetically.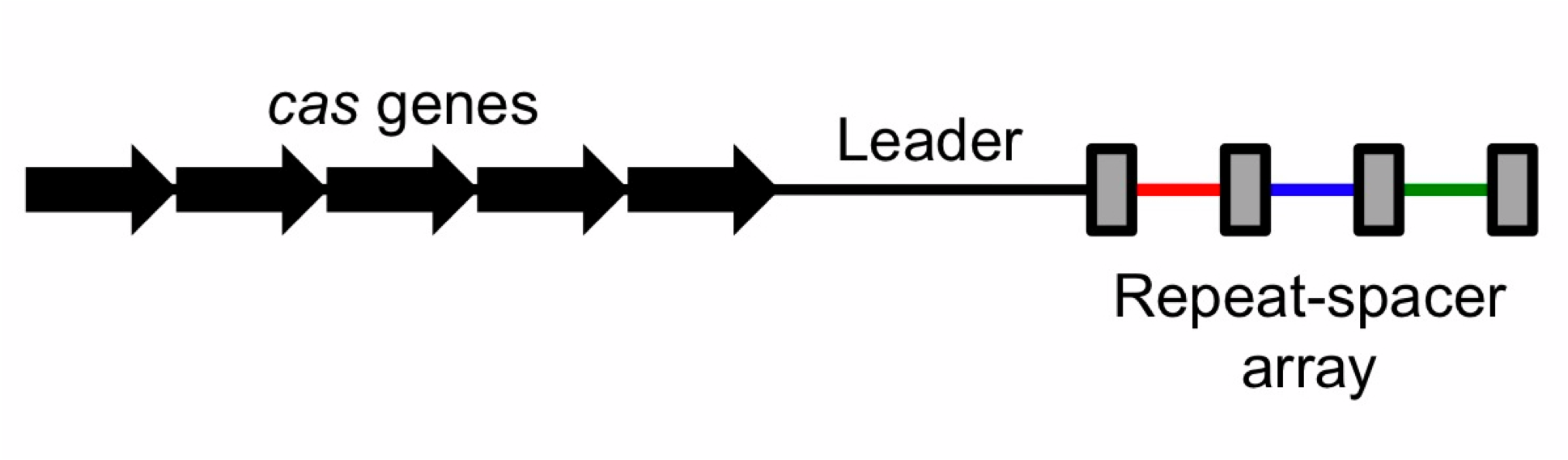 Diagram of CRISPR/Cas system. The repeats are displayed as rectangles. Spacers are colored. - cas3
- casABCDE12 (short for casA, casB, casC...)
What follows is the promoter of the CRIPSR locus, which has been sequenced to be A-T nucleotide rich. The CRISPR array of repeats and spacers completes the system.
Synthetic Version of CRISPR
The idea of our project comes from utilizing the E. coli bacteria's natural way of conferring resistance to foreign DNA and plasmid and see if it can be engineered such that the CRISPR/Cas system can target specifically any piece of DNA by means of programming the system with a spacer of any DNA sequence. Such a system has been engineered by the Laboratory of Microbiology in the University of Wageningen and is displayed below:
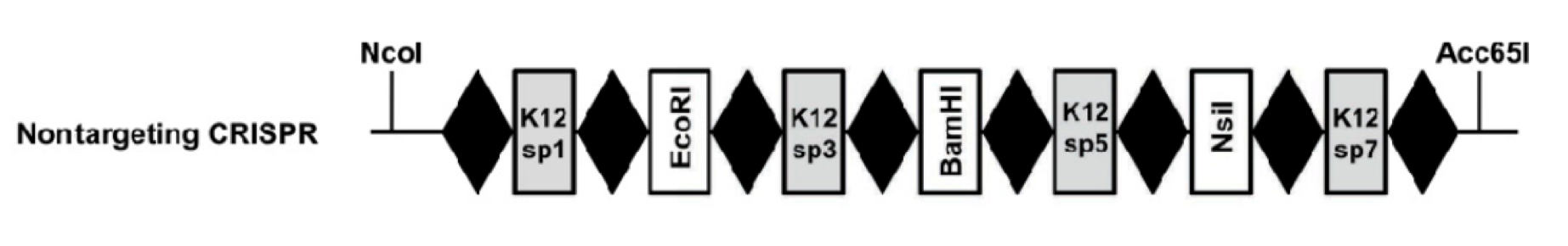 K12 Inspired CRISPR cassette with alternating K12-targeting spacers and restriction sites This part, BBa_K512002, is an IPTG-inducible CRISPR array genetically engineered with alternating spacers that target the K12 genome and contain restriction sites. The restriction enzyme sites are designed so that a spacer of any DNA sequence can be inserted into this CRISPR system.
In our particular project, we designed our synthetic CRISPR to target a DNA sequence coding for GFP. To do so, we took the synthetic CRISPR and GFP spacer and digested with two compatible restriction enzyme so that the spacer can be properly ligated into our synthetic CRISPR. The protocol is displayed below:
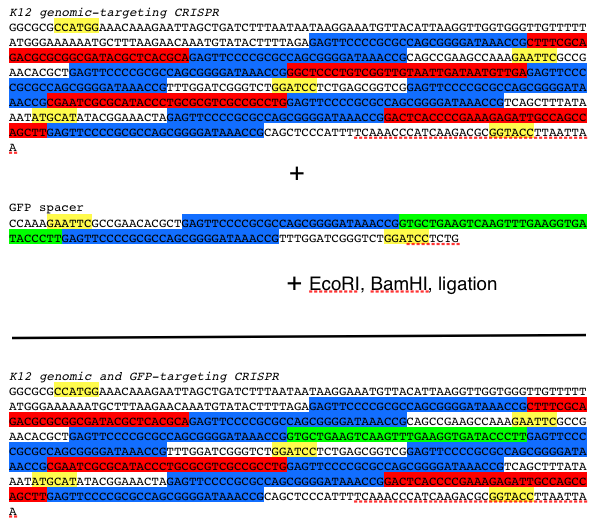 Protocol for constructing CRISPR-GFP: yellow=restriction enzyme site, blue=repeat, red=K12 targeting spacer, green=GFP-targeting spacer Results
Once the CRISPR-GFP plasmid has been constructed, we decided to test the system in K12 and B E. coli:
CRISPR/Cas System in K12 E. coli
To test the CRISPR/Cas system in K12, we designed the following strains:
- HT115 (K12) strain w/ tetO::GFP (AmpR)
- HT115 (K12) strain w/ tetO::GFP & CRISPR-GFP (AmpR & CmR)
- HT115 (K12) strain w/ CRISPR-GFP (CmR)
We tested each system by creating three batches for each strain so we can monitor the growth of the bacteria over time:
- One strain + antibiotics - IPTG
- Same strain + antibiotics + IPTG
- Same strain + antibiotics + 1 hr. before inducing system with IPTG
HT115 w/ tetO::GFP
Regarding the first strain (HT115 w/ tetO::GFP), we used this test as our control.
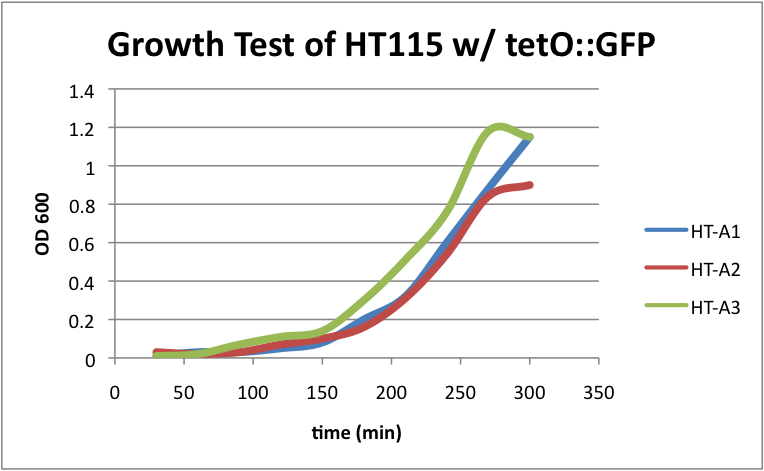 HT-A1=(-IPTG), HT-A2=(+IPTG), HT-A3=(1hr.+IPTG) The three batches of the strain grew at approximately the same rates.
HT115 w/ tetO::GFP & CRISPR-GFP
 Left: HT-B1 overnight; Right: HT-B2 overnight 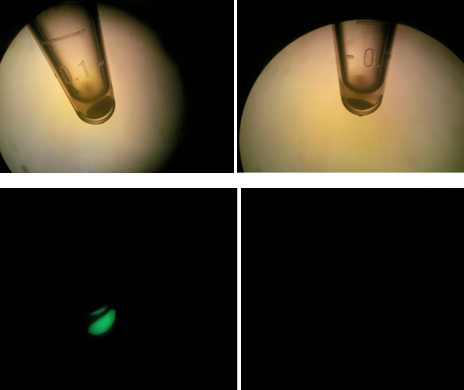 Upper & lower left: HT-B1; Upper & lower right: HT-B2 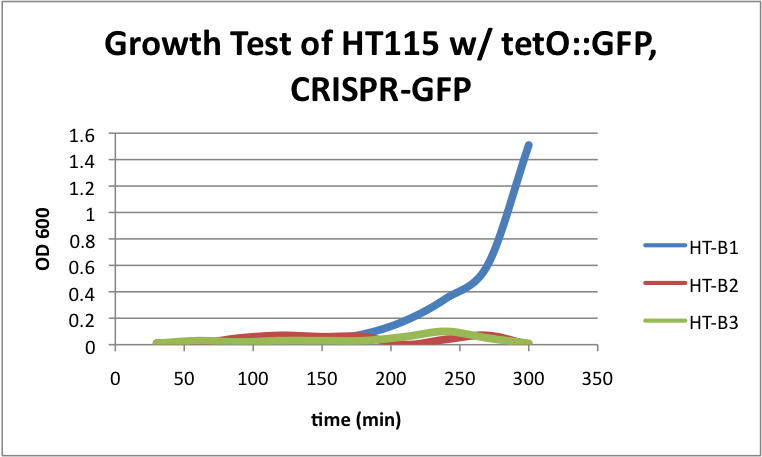 HT-B1=(-IPTG), HT-B2=(+IPTG), HT-B3=(1hr.+IPTG) With the addition of CRISPR-GFP, there is a severe drop off in growth rate for the batches induced with IPTG. This indicates that CRISPR-GFP system is destroying the tetO::GFP plasmid that also contains the antibiotic resistance gene for ampicillin. Since the bacteria are growing in cultures containing ampicillin, the activated CRISPR renders the bacteria incapable of surviving in the presence of ampicllin. As a result, they are no longer able to survive.
HT115 w/ CRISPR-GFP
 HT-D1=(-IPTG), HT-D2=(+IPTG), HT-D3=(1hr.+IPTG) This test is used to confirm that CRISPR can also target and destroy genomic DNA from the K12 family. Note that this CRISPR system also contains spacers that match the genomic DNA of K12 bacteria. Although this data introduces a bias to the growth test of strain HT115 with tetO::GFP & CRISPR-GFP, we are encouraged by the observation that the batches of bacterial strain HT115 with CRISPR-GFP induced by IPTG grew slightly more before dying from the presence of ampicillin.
CRISPR/cas System in B E. coli
To test the CRISPR/Cas system in B, we designed the following strains:
- BL21 (DE3) strain w/ tetO::GFP (AmpR)
- BL21 (DE3) strain w/ tetO::GFP & CRISPR-GFP (AmpR & CmR)
- BL21 (DE3) strain w/ tetO::GFP, CRISPR-GFP, cas3, casABCDE (AmpR, CmR, KanR, StrepR)
- BL21 (DE3) strain w/ tetO::GFP, CRISPR-GFP, cas3, casABCDE12 (AmpR, CmR, KanR, StrepR)
We tested each system by creating three batches for each strain so we can monitor the growth of the bacteria over time:
- One strain + antibiotics - IPTG
- Same strain + antibiotics + IPTG
- Same strain + antibiotics + 2 hr. before inducing system with IPTG
BL21 (DE3) strain w/ tetO::GFP
Regarding the first strain (BL21 w/ tetO::GFP), we used this test as our control.
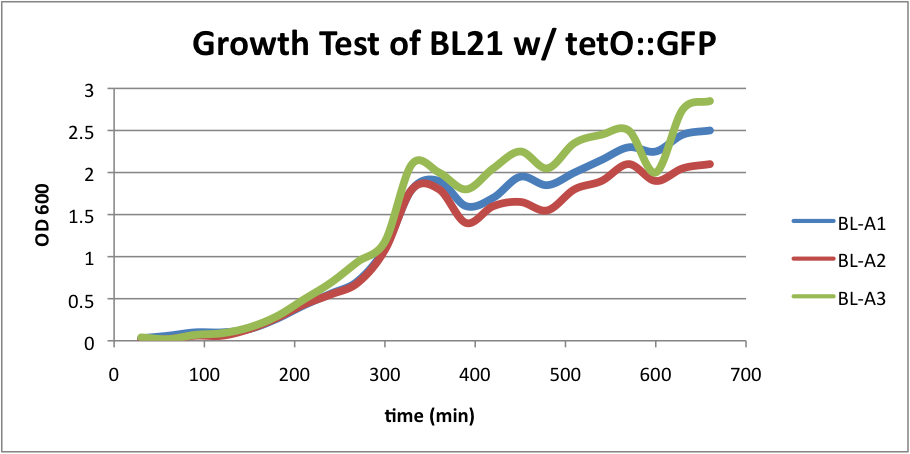 BL-A1=(-IPTG), BL-A2=(+IPTG), BL-A3=(2hr.+IPTG) The three batches of the strain grew at approximately the same rates.
BL21 (DE3) strain w/ tetO::GFP & CRISPR-GFP
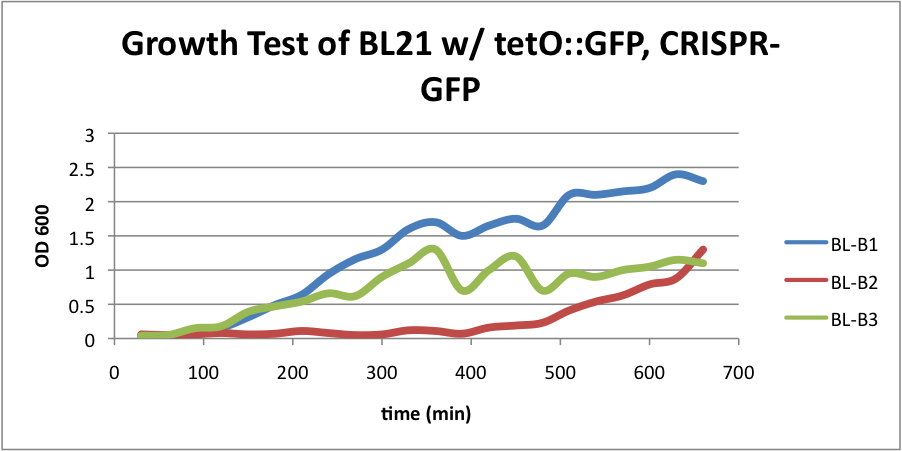 HT-B1=(-IPTG), HT-B2=(+IPTG), HT-B3=(2hr.+IPTG) BL21 (DE3) strain w/ tetO::GFP, CRISPR-GFP, cas3, casABCDE
BL21 (DE3) strain w/ tetO::GFP, CRISPR-GFP, cas3, casABCDE12
|