Team:Calgary/Project/Preliminary Data
From 2011.igem.org









Preliminary Testing

Background and Rationale
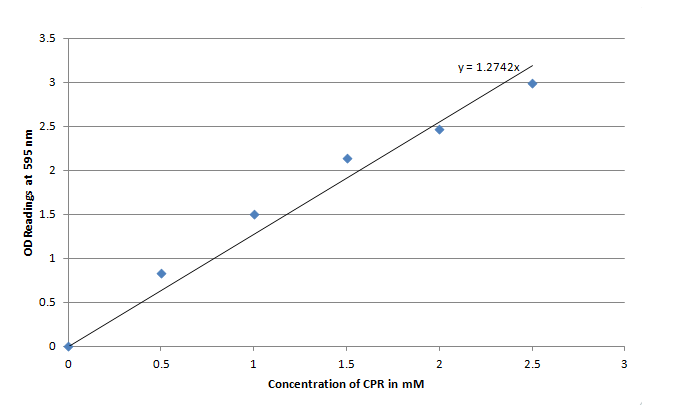
For the purposes of testing out the LacZ reporter as an electrochecmical detector it was important to first assess how well the reporter performed at cleaving the chlorophenol red β-D-galactopyranoside (CPRG) into Chlorophenol red (CPR). The LacZ gene that was chosen for this experiment as well as for the electrochemical testing was fused to an IPTG inducible promoter(LacI), or part I732901. Overall I732901 the purpose of the experiment was to ensure that the E. coli could be induced to produce β-galactosidase, which could then cleave chlorophenol red β-D-galactopyranoside (CPRG) to produce Chlorophenol red (CPR). Since CPR has a very dark characteristic red color at pH 7 the reaction wasto be kept at that constant pH using a phosphate buffer. This experiment was also expected to provide useful data for electrochemical side of things (i.e. oxidizing the CPR and measuring either the current or voltage) by determining what sort of time frame would be needed for the E. coli to produce a significant amount of CPR by cleavage of CPRG providing a guideline for future electrochemical testing.
Methods
E. coli carrying the plasmid were subcultured cultured until log phase then exposed to PBS buffer containing 3mM CPRG and 1mM IPTG for fixed lengths of time before measuring the absorbance of the solution. A detailed procedure are recorded in protocols section.
Results
Discussion of Results
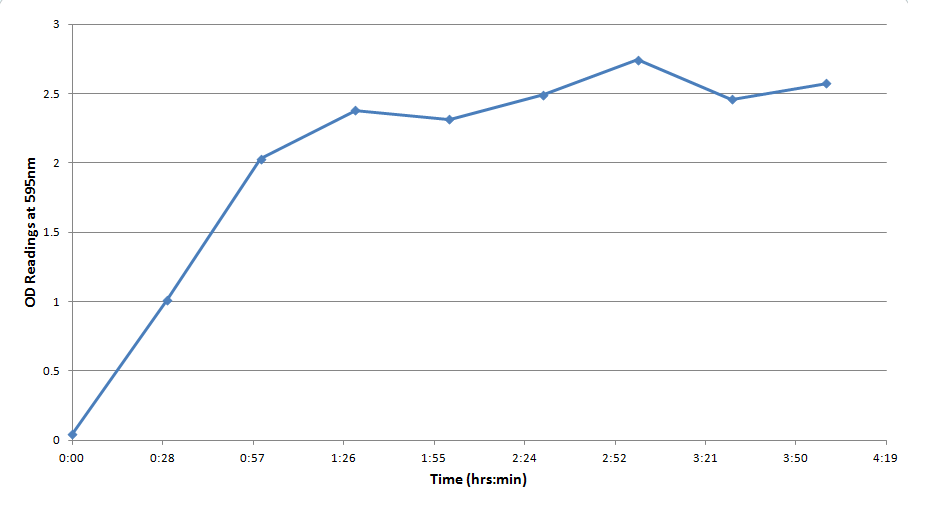
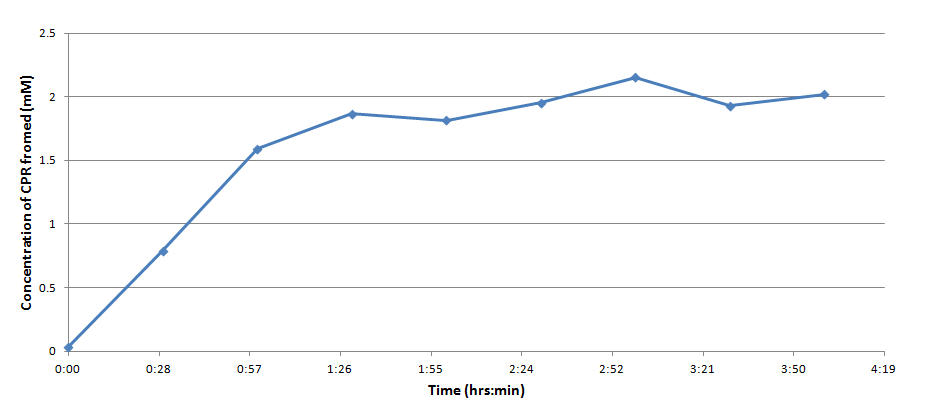
Figure 3 indicates that the concentration of CPR clearly increases over time in this mixture. Illustrating that the IPTG is successfully inducing the LacZ gene to produce β-galactosidase. This data will be useful to the electrochemical detector side of the project because they will now have data from which they can choose a time when they should begin oxidizing the CPR formed by the E. coli (by cleavage of CPRG).
One drawback experienced in this experiment was that the Victor plate reader which was used to take OD readings could not give data past an OD of ~3 for CPR absorption, meaning that even samples with an extremely high concentration of CPR would at best have an absorbance reading similar to a ~3 mM sample. Thus, this method was not particularly accurate for highly concentrated solutions of CPR. However the results did reveal that under the specified conditions about 1mM of CPR could be produced in around 30 minutes revealing that the system responded quite rapidly when induced with IPTG.

 "
"