Team:Berkeley/Parts
From 2011.igem.org
Revision as of 23:08, 27 September 2011 by Gabriellopez (Talk | contribs)



# of parts submitted:
Assembly Standard: BglBricks
Accomplishments:
- 35 Regulatory promoters
- 2 Ribosome Binding Sites
- 1 Reporter device
- 3 Composite expression modules
- 2 Coding proteins
Assembly Standard: BglBricks
Accomplishments:
- Our parts have been submitted to the registry and can be found HERE
- # of parts were physically sent to the registry
- # of parts were characterized in depth

Function
Stress promoters respond differently to various types of stress that is placed on the cell. We wanted a promoter that would be downregulated in the presence of membrane stress caused by the overexpression of ToxR fusion proteins. The design principle was to express ToxR fusion proteins under this promoter such that the stress it caused would downregulate its production.Assembly
The 34 stress promoters were PCRed from E. coli MC1061 Genomic DNA. Basic parts were made in plasmids with pUC origins. A constitutive promoter (Pcon) was also cloned into a basic part as a control for characterization.Characterization
The pool of 34 stress promoters, identified from microarray data (Moen, 2009), was assembled in front of a GFP reporter gene, and the construct was transformed into E. coli MC1061.

Experiments
The transformed cells were subjected to generalized stresses (heat, cold, acid, base, salt) overnight, and their resulting fluorescence was measured. Downregulation in response to stress, resulting in decreased fluorescence, was desired. Tecan plate measurements showed initial downregulation in some of the stress promoeters, with the cold condition yielding the best results. Further flow cytometry experiments confirmed the stress-responsive downregulation shown by the Tecan.


Function
The expression plasmid consists of a stress promoter regulating the production of ToxR. This construct allows us to express enough ToxR to allow for the functionality we desire and at the same time have minimal affect on bacterial growth rate.Assembly
The ToxR was PCR amplified out of V. Cholerae genomic DNA, digested, and ligated in front of the leucine zipper (Iilk.) A library of potential stress promoters and an RBS library were put in front of the ToxR fusion protein and the library was screened for healthy and transcriptionally active clones. This construct was then used as our template for our other ToxR fusion proteins.
Characterization
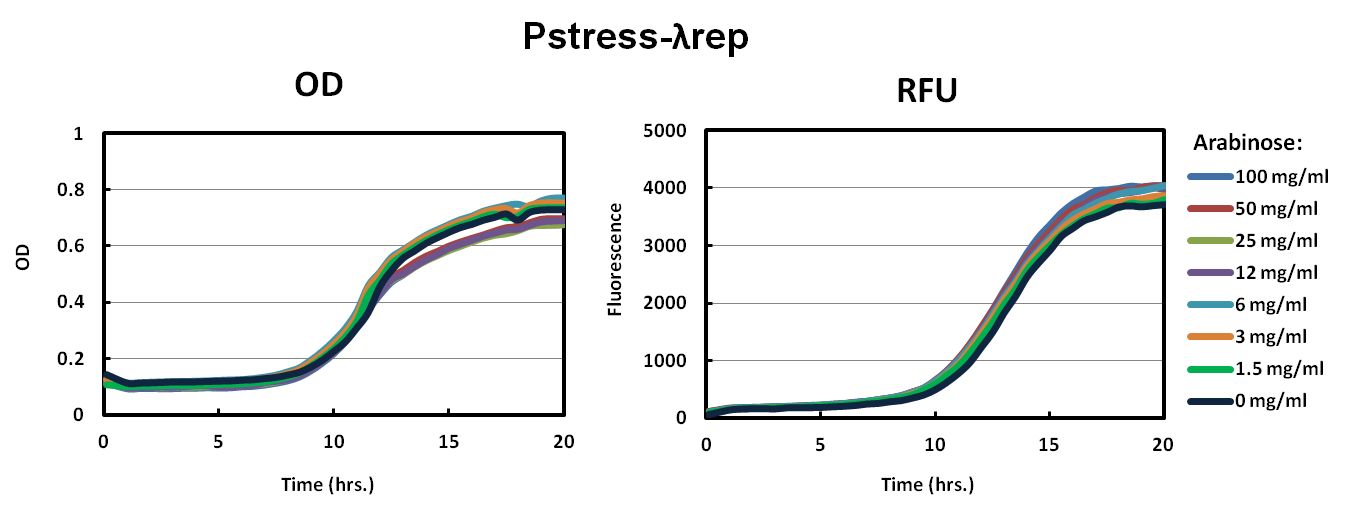
We characterized our stress promoters by transforming ToxR-LambdaRep driven by Pstress into our reporter strain. In parallel, we also transformed our original constructs of ToxR-lambdaRep driven by Pbad into our reporter strains. Each construct was grown in 100 mg/ml, 50 mg/ml, 25 mg/ml, 12 mg/ml, 6 mg/ml 3 mg/ml, 1.5 mg/ml, and 0 mg/ml arabinose. The control for our characterization experiment is a known nontoxic plasmid that should not activate our reporter strain.As shown in the graph of Pbad Lambdarep and Pbad MukF, cells did not grow up well in the varying levels of arabinose. Similar to the results, the relative fluorescence supported the conclusion that our constructs were toxic to the cells.
Our solution to the high toxicity of our ToxR chimeras was to have a promoter that would down regulate transcription when the cell was under stress. As shown in the Pstress graphs of Lambdarep and MukF, cells grew to a much higher OD and fluorescence especially in Pstress LambdaRep compared to the control. All levels of arabinose showed similar results.
Experiments





Function
The reporter construct will give us a fluorescent output that will correlate to the amount of transcriptional activity ToxR promotes on pctx. Since transcriptional activity by ToxR dimers depends on the strength of dimerization, the fluorescence can be correlated to how tight the ToxR monomers bind together.Assembly
ToxR’s native target promoter ctx was PCRed out of V. Cholerae genomic DNA and made into a basic part. The promoter was then placed upstream of an rbs.ffGFP part using 2AB assembly.
Characterization
By cotransforming this reporter plasmid and the expression plasmid (see above) we were able to measure the strength of Pctx induction by ToxR.Experiments
GRAPH!!!!
Function
The human estrogen receptor is known to dimerize around estradiol. By fusing the human estrogen receptor to ToxR, we can create an estradiol biosensor and potentially an estradiol-inducible system.Assembly
The estrogen receptor was refactored to optimally express in E. Coli and assembled using designed oligos and PCA (polymerase cycling assembly). The final protein was made into a basic part. Various sections of the estrogen receptor were PCRed into separate basic parts to create truncations that might give different behaviors to estradiol (see figure). The estrogen receptor was fused to ToxR’s C-terminus with Gly-Ser linkers and thus exposed to the periplasm when expressed in the cell.
Characterization

 "
"