July 24
Started overnight cultures of pSB3K3
July 25
PCR'd parts for pNT002: R0040, K123001, B0014, pSB4A5
PCR'd GFP control insert and pNT003 insert
Gibson assembly and transformation of pNT003 with a negative control
Nanodrop of evaporation concentrated PFGE DNA: 9-1: 11.4ng/uL; 10-2: 29.7ng/uL
Attempt at packaging above DNA with fosmid kit extract, but enzyme wasn't heat inactivated as par procedure
50 mL of phage dillution buffer made for fosmid kit
Evaporation concentrated more PFGE DNA. Nandrop results: 9mix: 14.3ng/uL; 10mix: 2.2ng/uL
Overnight ligation of 9mix and (10mix + 10-2) to redo fosmid packaging, at 16°C
Results
Decided not to use the pNT003 PCR'd insert, as the band of the correct length was much fainter than a lower length band. Chose not to do Gibson assembly of the positive GFP control, despite not having any green colonies last week, instead focusing on assembling pNT002 and pNT003.
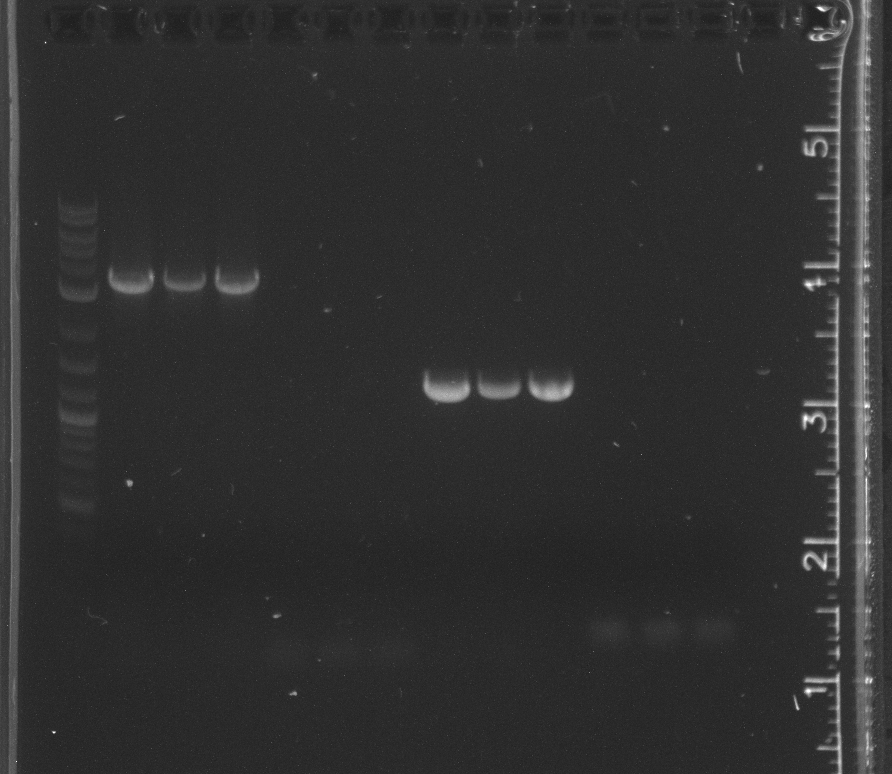
lane 1 NEB 2-log ladder, 2-4 pSB4A5, 5-7 R0040, 8-10 K123001, 11-13 B0014
|
lane 1 NEB 2-log ladder, 2-4 pNT003 insert, 5 R0010, 6 K123003, 7 B0014, 8 blank, 9-11 GFP insert, 12 GFP constitutive promoter, 13 GFP, 14 GFP terminator, 15 NEB 2-log ladder
|
PCR concentrations
| Part |
Concentration (ng/ul) |
| R0040 for pNT002 |
56.4 |
| K123001 |
121.5 |
| B0014 for pNT002 |
100.8 |
| pSB4A5-1 |
98.0 |
| pSB4A5-2 |
97.4 |
PSB3K3 miniprep concentrations
| Part |
Concentration (ng/ul) |
| 1 |
36.2 |
| 2 |
23.6 |
| 3 |
10.6 |
| 4 |
17.7 |
| 5 |
20.3 |
July 26
Packaging of 9mix and (10mix + 10-2) ligations
Attempted various methods of spreading chemical solution on minimal media plates
16s PCR
Ran 20 ul Gibson reaction of pNT003 and a negative control. PCR purified these using the Qiaquick kit. Ran an Spe1 digest on part of the reaction. If the plasmid had formed, a cut with Spe1 would create a band of around 5kb.
Plated enrichment cultures on LB
Results
| Part |
Concentration (ng/ul) |
| pNT003 + |
27.8 |
| pNT003 - |
23.4 |
The gel gave no bands in any lane, with or without the restriction digest. pNT003 + without the Spe1 digest had a faint smear, the other lanes had nothing. Chose to transform anyway
All enrichment cultures but one showed growth on LB.
|
|
17a-ethinylestradiol no V
|
17a-ethinylestradiol no V
|
|
|
|
|
|
|
|
|
BPA room temperature +V location 1
|
BPA room temperature +V location 2
|
BPA room temperature +V location 3
|
BPA room temperature +V location 4
|
BPA room temperature no V location 1
|
BPA noV roomT 2.jpg
BPA room temperature no V location 2
|
BPA room temperature no V location 3
|
BPA room temperature no V location 4
|
|
|
|
|
|
|
|
|
July 27
Grew up cells for titering of packaged fosmids; reached OD600 of 0.974
Redid 16s PCR
Attempted to PCR the inserts for pNT002 and pNT003 together from their component parts. Did a gel extraction to get the correct length product
PCR pSB3K3 to linearizer it for Gibson assembly
Continued attempts to spread chemical solution on minimal media plates
Minimal media transfers
Results
lane 1 NEB 2-log ladder, 2-3 negative controls, 4-6 LA River sample 9, 7-9 LA River sample 10, 11-12 pET53DEST
|
lane 1 NEB 2-log ladder, 2-4 pSB3K3
|
The 16s PCR from yesterday, shown in the gel today, had a band in the first lane of negative controls, indicating contamination.
The pSB3K3 amplification failed. The bands indicated a much lower length than expected. After doing an alignment in Geneious, it appears our pSB3N5 primers we ordered for pSB3C5, which we are no longer using because of our competent cell strain, do not work with earlier versions of the 3 origin plasmids.
Both the negative control and experimental of PCR purified Gisbson assembly from yesterday of pNT003 had 0 colonies. The transformation with 1 ul pUC 19 had no colonies.
| Part |
Concentration (ng/ul) |
| pNT002 insert |
28.3 |
| pNT003 insert |
9.5 |
BPA solutions fail to form uniform layers when poured on top of agar, but form a uniform layer when poured directly onto a clean plate.
July 28
Plate packaged fosmids to obtain titer
Set up enzyme binding assay of p450s with 17-α estradiol, bispehnol A, and nonylphenol with cytochrome p450-BM3 enzymes WT-F87A, H2A10, and 9-10ATSF87A
Try out plating chemicals on bottom of plates with agar bacteria suspension on top
Miniprepped pSB4A5 from overnight culture of glycerol stock-54 ng/ul
Set up restriction digests of pNT002 insert and pSB3K3-1 with EcoRI-HF and PstI according to NEB protocol
Ligated pNT002 and pSB3K3 following NEB protocol
Find positive control for transformation, test transformation
Run gel of 16s and vector. Dpn1 digest vector.
Attempt to PCR pNT003 insert and gel extract it
Results
Yesterday's 16s PCR failed, as there were no bands in control or experimental lanes. The vector was amplified and was the correct length. We redid it, but again there were no bands in the control or experimental lanes.
Enrichment cultures plated in minimal media with chemical layer, to be checked in a few days.
Gel extraction of pNT003 insert was not much better than yesterday. Here are the concentrations:
| Part |
Concentration (ng/ul) |
| pNT003 insert 1 |
8.0 |
| pNT003 insert |
3.7 |
July 29
Attempted to PCR pNT002 and pNT003 inserts from the purified gel extractions of July 27, using the same primers.
Attempted to PCR pNT003 insert from component parts with a gradient of DMSO to prevent non-specific priming and hopefully amplify the correct length insert instead of the 400bp band that keeps appearing.
Titering of packaged fosmid inserts at concentrations 1x, .1x, .01x, and .001x on LB-chlor plates
Analysis of p450 assay with reverse-phase HPLC
Tried longer ligation time for pNT002 insert/pSB3K3
Results
HPLC reveals shift in the chemical structure of BPA after incubation with p450's WT-F87A and 9-10ATSF87A from a peak of 5.26 to 6.5
HPLC UV peak for BPA dissolved in DMSO
|
HPLC UV peak for BPA degraded by WT-F87A (a p450 enzyme)
|
9-10ATSF87A.jpg
HPLC UV peak for BPA degraded by 9-10ATSF87A (a p450 enzyme)
|
No colonies on pNT002/pSB3K3 ligation
July 30
Take out plates from fosmid titering
Ran gels of PCR of pNT002 insert, pNT003 insert and pSB3K3 from yesterday.
Mass spectroscopy of bisphenol A's p450 degradation products
Results
Two colonies on both 9mix and 10-2mix fosmid 1x dilutions
lane 1 NEB 2-log ladder, 2-4 pNT002 insert, 5-7 pNT003 insert, 8-10 pSB3K3
|
PCR of pNT003 insert with increasing DMSO:lane 1 NEB 2-log ladder, 2-3 0% DMSO, 4-5 0.5%, 6-7 1%, 8-9 2%, 10-11 3%
|
pSB3K3 was not amplified. The DMSO does not to appear to have decreased the 400 bp band or increased the correct 2 kb band.
No colonies from pNT002/pSB3K3 ligation.
BPA samples did not ionize well in LCMS; retry with GCMS.
 "
"