Team:NTNU Trondheim/Characterization
From 2011.igem.org
m (→ppGpp's effect on rrnB P1 promoter) |
m (→ppGpp's effect on rrnB P1 promoter) |
||
| Line 3: | Line 3: | ||
==ppGpp's effect on rrnB P1 promoter== | ==ppGpp's effect on rrnB P1 promoter== | ||
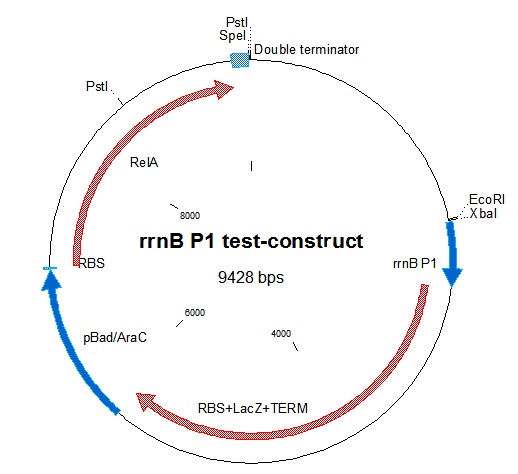
| - | [[File:RrnB-relA2.jpg| | + | [[File:RrnB-relA2.jpg|thumb|Figure 1: Plasmid map of the rrnB P1 test-construct. Note the PstI site in RelA.]] |
Latest revision as of 17:58, 31 August 2011

ppGpp's effect on rrnB P1 promoter
Abstract
To test the down-regulating effect of ppGpp on rrnB P1, which is the basis of our project, we decided to make a construct containing ppGpp Synthase (RelA) inducible by the pBAD/AraC promoter. The construct would also contain beta-galactosidase (lacZ) which would be expressed by the rrnB P1 promoter. Thus, induction of the pBAD/AraC promoter with arabinose should give lower lacZ production, as relA is overproduced giving high ppGpp concentration. We were not able to show this effect in our experiment.
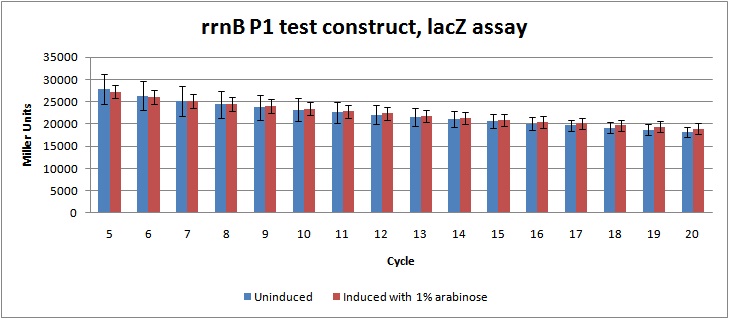
Figure 2: LacZ assay data in Miller units from rrnB P1 test-construct, cycle 5-20. Showing no significant difference between the induced cultures (which should be lower due to prduction of relA) and uninduced cultures.
Unfortunately, the data from the assay indicates no significant regulation of the rrnB P1 promoter, as there is no difference between the induced and the uninduced cultures. This might be due to problems during the genetic construction, as enzyme test cutting of the construct didn't give the expected fragments. The construct will be sequenced to figure out what went wrong.
Another problem was that it seemed like the lacZ construct had constitutive expression without a promoter. The transformants containing only the [http://partsregistry.org/Part:BBa_I732019 lacZ BioBrick] gave blue colonies on X-gal, and analysis of the BioBricks sequence showed promoter-like features ahead of the actual lacZ gene in some web-tools for promoter analysis. As the rrnB P1 promoter is quite weak in our constructs (see stress-sensor), it could be that the difference in rrnB P1 expression is over-shadowed by the constitutive expression of the lacZ BioBrick.
ONPG assay
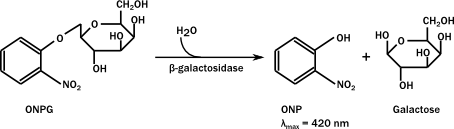
β-galactosidase hydrolyzes ortho-Nitrophenyl-β-galactoside (ONPG) to ONP + galactoside. ONP gives a yellow color, and absorbs at 420 nm. LacZ assays are widely used to test promoter activity. Here, the assay was performed as follows;
Cells were grown over night in pre-culture, inoculated 1% in appropriate media in 3 parallells and grown for 18 hours. 5µL culture was transferred to a 96 well plate and 100 μl of Z-buffer with chloroform (Z-buffer: 0,06 M Na2HPO4 x 7H20, 0,04 M NaH2PO4 x H20, 0,1M KCl, 0,001 M MgSO4x7H2O, pH 7; Z-buffer with chloroform: Z-buffer, 1% β-mercaptoethanol, 10% chloroform)was added. 50 µL Z-buffer with SDS 1,6% was added to lyse the cells, and the plate was incubated for 10 minutes in room temperature. 50 µL 0,4 % ONPG solution in Z-buffer was added, and OD405 was measured with 1 minute intervals for 20 cycles, in 4 parallells.
Miller units were calculated as shown in [http://openwetware.org/wiki/Beta-Galactosidase_Assay_(A_better_Miller) Openwetware's A better Miller], to compensate for OD and reaction time. Figure 2 shows the Miller units from cycle 5-20.
Genetic construction
Our relA BioBrick was digested with E+S, it was ligated into E+X-digested [http://partsregistry.org/partsdb/get_part.cgi?part=BBa_B0015 BBa_B0015]. the relA+double terminator was then ligated into an RBS backbone from [http://partsregistry.org/wiki/index.php/Part:BBa_B0034 BBa_B0034]. To be able to control the expression of relA, the arabinose-inducible promoter/inducer [http://partsregistry.org/Part:BBa_K113009 BBa_K113009] containing pBAD/AraC was put in front of the construct.
rrnB P1 was joined with [http://partsregistry.org/Part:BBa_I732019 BBa_I732019] containing an RBS, lacZ coding for beta-galactosidase, and a double terminator. This was then combined with the pBAD-relA construct. A problem we encountered was that relA contains a PstI site at bp 1393. This was mostly worked around in our construction. In one step in the construction, we had to digest relA with PstI. In that case, partial digestion was used. The final construct is represented in figure 1.
(1) Tedin, K., A. Witte, et al. (1995). "Evaluation of the E. coli ribosomal rrnB P1 promoter and phage-derived lysis genes for the use in a biological containment system: A concept study." Journal of Biotechnology 39(2): 137-148.
 "
"