Team:KAIST-Korea/Projects/Modeling/Section5
From 2011.igem.org
(Difference between revisions)
| Line 20: | Line 20: | ||
padding-bottom:9px; | padding-bottom:9px; | ||
text-align:center; | text-align:center; | ||
| - | text-decoration; | + | text-decoration:none; |
text-transform:uppercase; | text-transform:uppercase; | ||
} | } | ||
Revision as of 15:25, 12 August 2011
E.Casso computer simulation
In order to predict the behavior of the real drawing system, we ran a computer simulation before conducting the actual experiment in the wet lab. The program takes into account the conclusions and assumptions established so far to produce consecutive frames depicting the progress of the drawing in chronological order.
The goal is to accurately predict the expected colorwise random painting produced for a given distribution of the two types of genetically modified E.coli over a given period of time.
Modeling Approach
Assumption Made
- From report 1: Quorum production by the Brush E.coli, we know that 56 quorum molecules are produced per minute. Although they are produced continuously in reality, we assumed that they appear altogether at the end of each minute for the purpose of simplifying the modeling procedure; it is difficult to capture the dynamics of a continuous production of quorum molecules in discrete, consecutive frames. To further simplify the procedure, we assumed that the frames are separated by a one minute time interval, which is compatible with the number of quorum molecules produced during the same time interval as determined in report 1.
- We also assumed the petri dish as a two dimensional grid consisting of ordered squares of side length 1.1 micro-meter as this configuration approximates the micro-scale arrangement of E.coli with reasonable accuracy (report 2: Quorum Diffusion). The conclusions of this report also justify our assumption that quorum molecules diffuse sufficiently fast that we can assume that neighboring cells receive quorum at the end of each minute.
- From report 3: Fluorescence production by the paint E.coli, we confirmed that fluorescent proteins are indeed produced by the paint E.coli upon receiving quorum.
- In report 4: Fluorescence visibility justification, we observed that noticeable fluorescence builds up even if as little as only three out of a thousand adjacent cells are successfully induced by IPTG. From this result, we conclude that fluorescence is readily observable upon production of fluorescent proteins. Consequently, we assumed that each cell in our grid definitely displays colors in response to induction.
Program input
- The user provides the program with the concentrations of the two types of E.coli, one that produces quorum and another that responds by producing fluorescent proteins, the dimensions of the medium, which in this case serves as the canvas, and a sketch of the picture by either directly moving the cursor around on the screen (intentional seeding) or by employing the random seeding function which randomly scatters both types of E.coli across the canvas (random seeding).
- Each method of seeding displays distinct characteristics. As mentioned earlier, random seeding randomly scatters both types of E.coli across the canvas, which results in most cases a conglomeration of colors uniformly spanning the canvas. On the other hand, intentional seeding allows the user to draw specific figures or delineate curvatures as he wishes. Because the amount of E.coli dropped onto the canvas is proportional to the time until the mouse is unclicked, he can choose to seed heavily or lightly by controlling the mouse appropriately. Lastly, the higher the concentration provided to the program, the heavier the default seeding.
Algorithm
- In each frame, the following series of steps take place. First, Brush E.colis produce quorums, which diffuse to adjacent cells. As established earlier, quorum diffuses sufficiently fast to adjacent cells that we can guarantee it diffuses to neighboring cells well before the advent of the next frame. Upon receiving quorum, paint E.colis produce quorum and fluorescent proteins, whose expression is modeled in our program as solid RGB colors occupying the area of each cell. The previous series of steps constitute all the components contained within a frame. The process repeats every minute, which is a repetition of the same process described here.
Output
- The program will save and display the resultant randomly generated color picture over a user-specified time period. It will also store the progress of the effect of quorum sensing and fluorescent protein production in discrete, chronologically consecutive frames. It also saves and displays the distribution of each type of E.coli across the canvas.
Results & Analysis
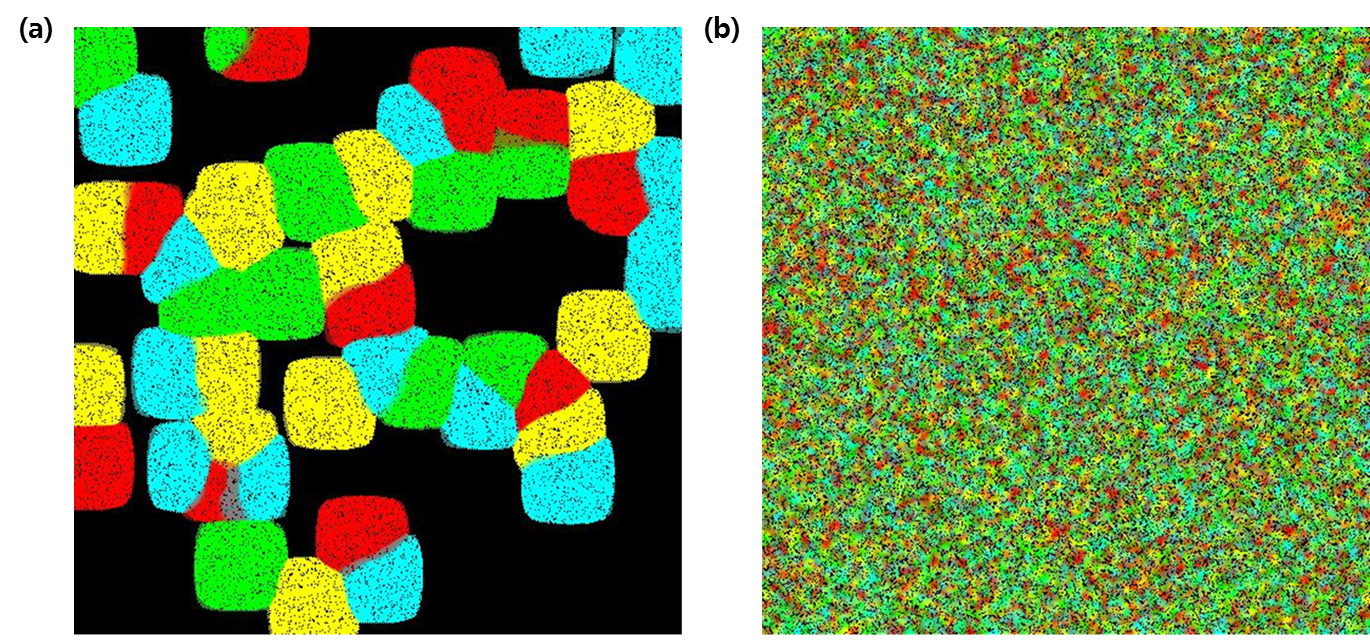
- Figure 2 displays the paintings obtained by randomly seeding 50 Brush E.coli and 200,000 paint E.coli (Fig 2. a) and 30,000 Brush E.coli and 150,000 paint E.coli (Fig 2. b) across the canvas. We obviously observe a difference in the density and uniformity of colors between the two pictures. In the Fig 2. a, distinct colors are clearly visible as Brush E.colis outnumbered the paint E.colis; borderlines between colonies expressing the same colors are clearly delineated. On the contrary, the painting on the right is chaotic; there are no clear colors visible but instead the entire canvas is a uniform mixture of the four colors. Of course, this is expected since the number of E.coli opting to “expand its own colorful empire” is now comparable to that of the paint E.coli.
- Figure 3 shows the result of intentional seeding. In the left, the user moved and clicked the mouse in the shape of iGEM. In the right, KAIST was written. The distributions of each type of E. coli are displayed above each painting.
- If you want to see more detailed results, check out the <'Simulation Results Gallery'>
 "
"