Team:Caltech/Week 8
From 2011.igem.org
| Line 10: | Line 10: | ||
PCR pSB3K3 and pNT003 insert with the new primers.<br/> | PCR pSB3K3 and pNT003 insert with the new primers.<br/> | ||
Gel extract the pNT002 insert from the July 29 PCR<br/> | Gel extract the pNT002 insert from the July 29 PCR<br/> | ||
| + | Standard Assembly Digest: K123001, B0014, B0034, R0010, PSB4A5 <br/> | ||
Minimal media transfers<br/> | Minimal media transfers<br/> | ||
Transformation and plating of pUC19 into competent cells for cell dry weight experiment<br/> | Transformation and plating of pUC19 into competent cells for cell dry weight experiment<br/> | ||
| Line 19: | Line 20: | ||
No colonies visible on the pUC19-transformed cells<br/> | No colonies visible on the pUC19-transformed cells<br/> | ||
<p>The enrichment culture plates show no visible growth yet.</p> | <p>The enrichment culture plates show no visible growth yet.</p> | ||
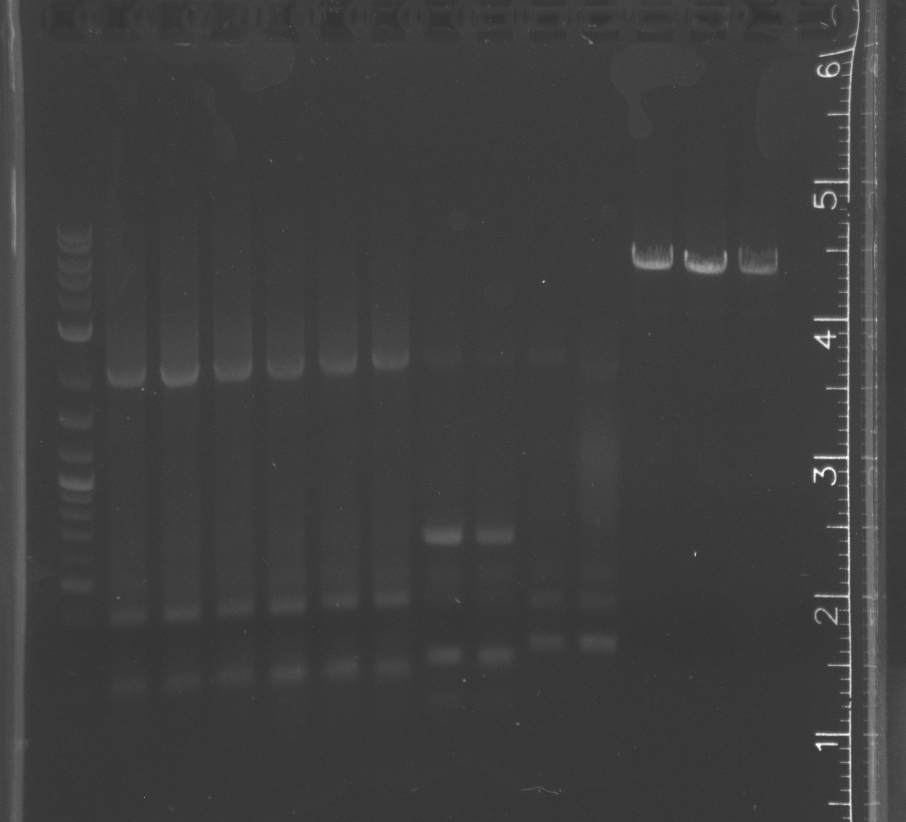
| + | Gel showed that the terminator, RBS, and lac promoter did not digest for standard assembly. <br/> | ||
Gel Extractions of July 29 PCR of pNT002 and pNT003 inserts | Gel Extractions of July 29 PCR of pNT002 and pNT003 inserts | ||
<table border="1"> | <table border="1"> | ||
| Line 69: | Line 71: | ||
==August 3== | ==August 3== | ||
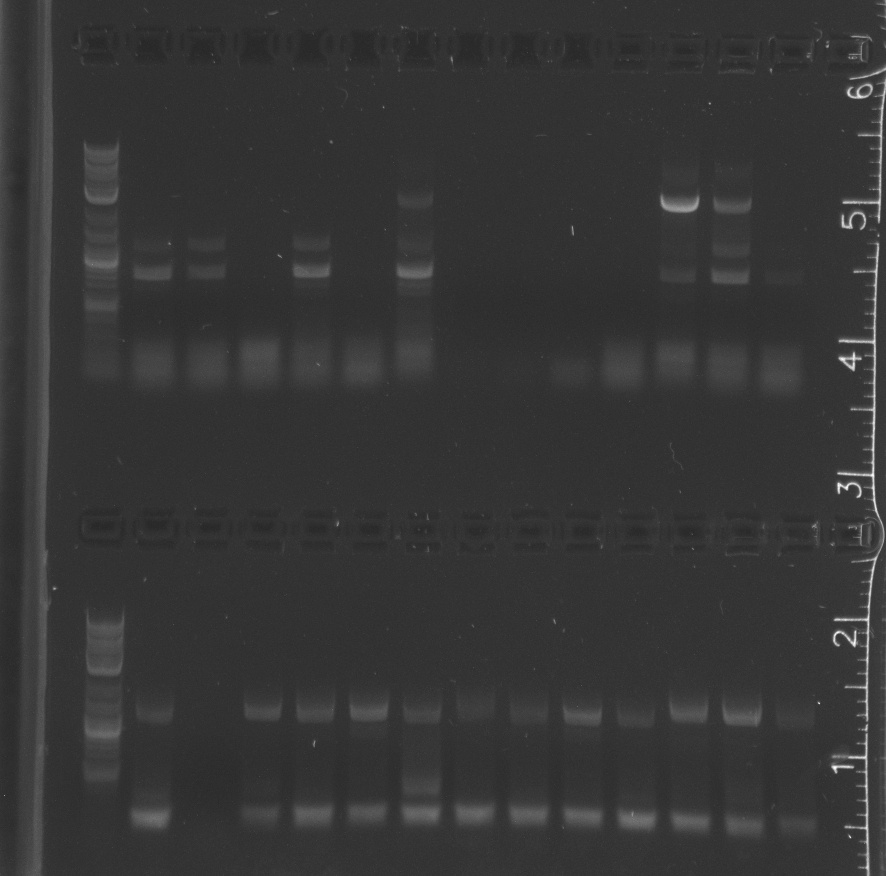
Colony PCR of Gibson transforms<br/> | Colony PCR of Gibson transforms<br/> | ||
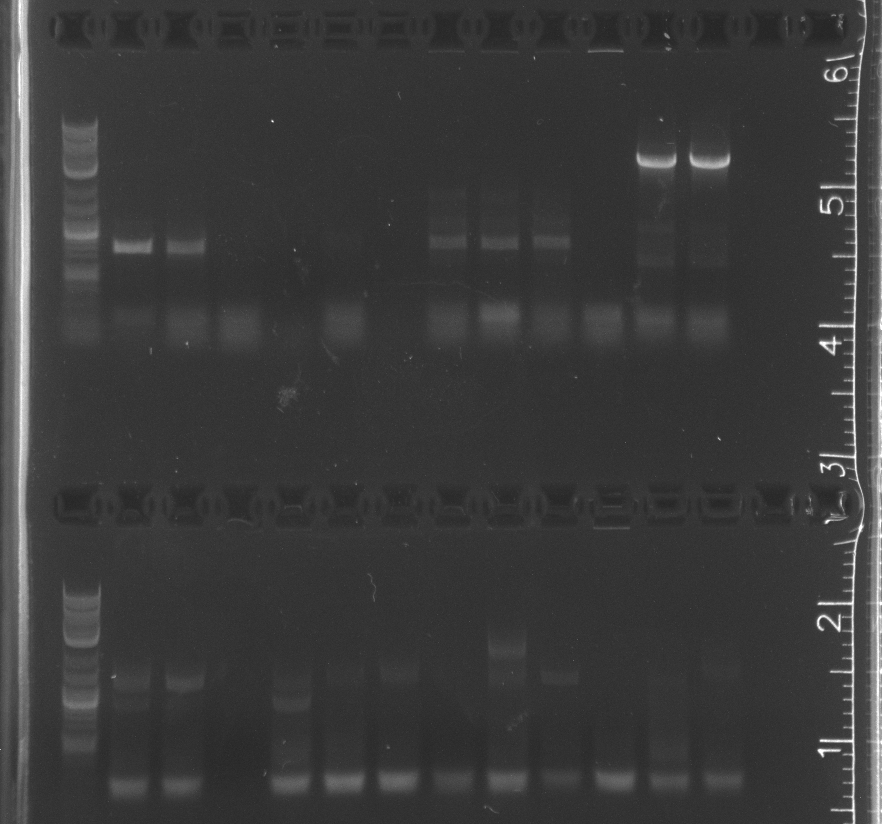
| + | Another assembly digest with gel extraction.<br/> | ||
Emzo's protocol for traditional assembly of PCR'd pNT002 insert and pSB3K3 plasmid<br/> | Emzo's protocol for traditional assembly of PCR'd pNT002 insert and pSB3K3 plasmid<br/> | ||
===Results=== | ===Results=== | ||
| Line 109: | Line 112: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| + | |||
| + | Only the enzyme and the vector had correct band lengths on the gel. We extracted them and nanodropped, but concentrations too low.<br/> | ||
| + | |||
==August 4== | ==August 4== | ||
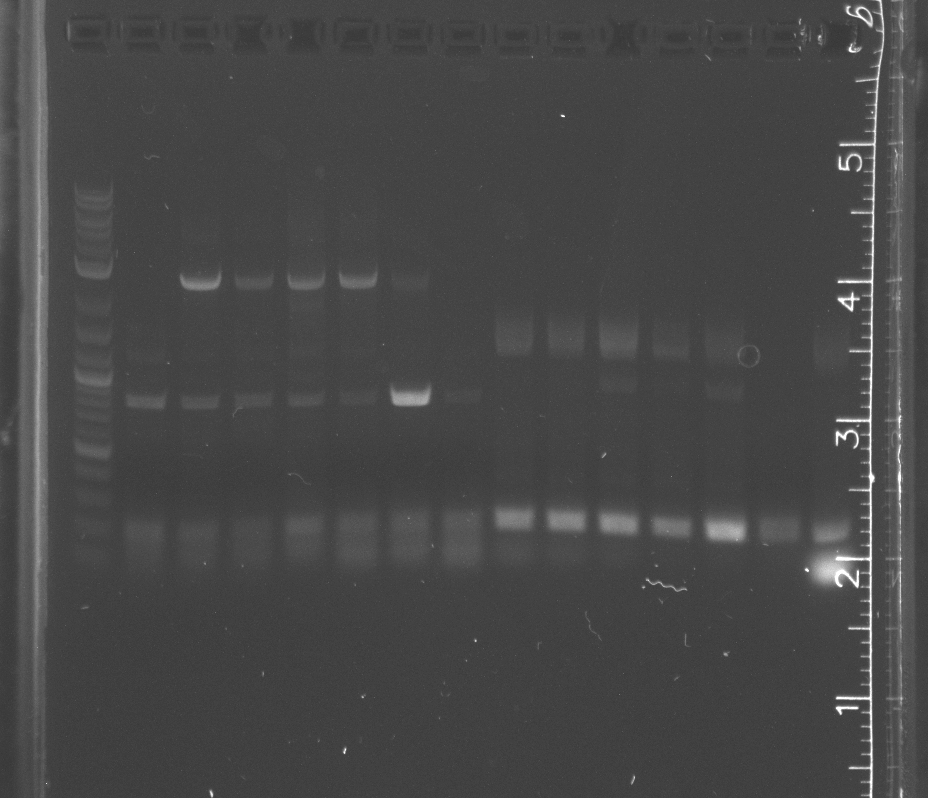
Ran gel of colony pcr from yesterday<br/> | Ran gel of colony pcr from yesterday<br/> | ||
Revision as of 18:57, 5 August 2011
|
Project |
July 31Started a LB+amp overnight culture of the B0034 glycerol stock from the 2010 Caltech iGEM Team ResultsMass spectrometry did not run correctly; samples did not ionize; must rerun August 1PCR pSB3K3 and pNT003 insert with the new primers. ResultsNo colonies visible on the pUC19-transformed cells The enrichment culture plates show no visible growth yet. Gel showed that the terminator, RBS, and lac promoter did not digest for standard assembly.
August 2Gibson assemble pNT002 using the gel extracted insert and linearized pSB3K3 ResultspNT002 ligation showed band on gel
August 3Colony PCR of Gibson transforms Results
Only the enzyme and the vector had correct band lengths on the gel. We extracted them and nanodropped, but concentrations too low. August 4Ran gel of colony pcr from yesterday ResultsSome of the colony PCR have insert amplifications of the correct length. Will send some off for sequencing tomorrow. No growth from traditional assembly on self ligation control or experimental plate
|
 "
"