Team:Caltech/Week 7
From 2011.igem.org
| Line 138: | Line 138: | ||
Continued attempts to spread chemical solution on minimal media plates<br/> | Continued attempts to spread chemical solution on minimal media plates<br/> | ||
Minimal media transfers <br/> | Minimal media transfers <br/> | ||
| + | |||
===Results=== | ===Results=== | ||
| + | |||
<gallery> | <gallery> | ||
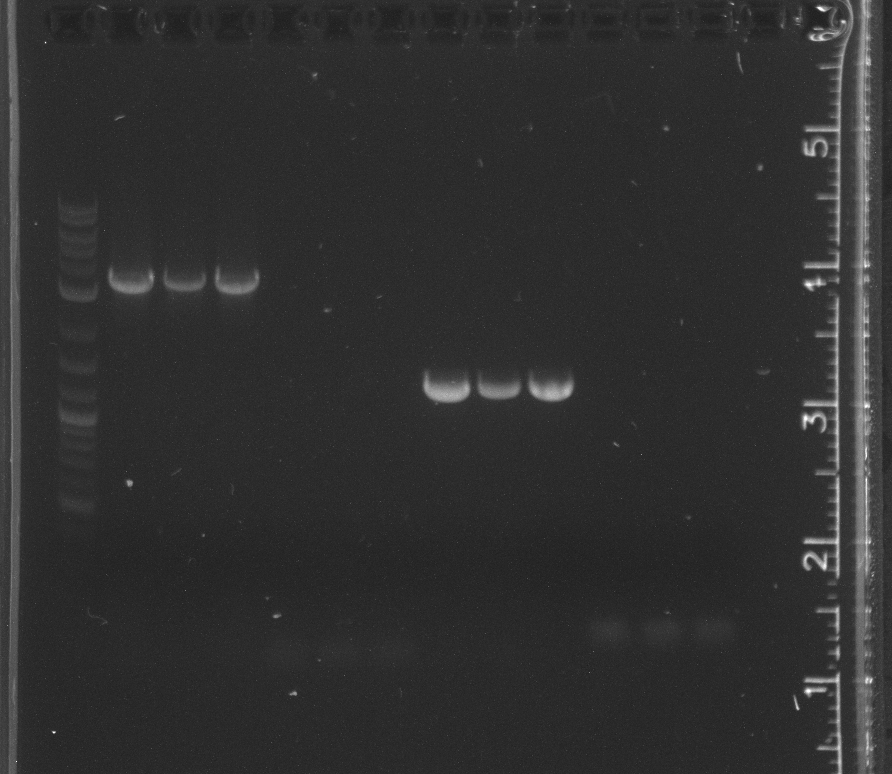
File:7-2716sgel.jpg|lane 1 NEB 2-log ladder, 2-3 negative controls, 4-6 LA River sample 9, 7-9 LA River sample 10, 11-12 pET53DEST | File:7-2716sgel.jpg|lane 1 NEB 2-log ladder, 2-3 negative controls, 4-6 LA River sample 9, 7-9 LA River sample 10, 11-12 pET53DEST | ||
File:7-27psb3k3.jpg|lane 1 NEB 2-log ladder, 2-4 pSB3K3 | File:7-27psb3k3.jpg|lane 1 NEB 2-log ladder, 2-4 pSB3K3 | ||
</gallery> | </gallery> | ||
| + | |||
The 16s PCR from yesterday, shown in the gel today, had a band in the first lane of negative controls, indicating contamination.<br/> | The 16s PCR from yesterday, shown in the gel today, had a band in the first lane of negative controls, indicating contamination.<br/> | ||
| + | |||
The pSB3K3 amplification failed. The bands indicated a much lower length than expected. After doing an alignment in Geneious, it appears our pSB3N5 primers we ordered for pSB3C5, which we are no longer using because of our competent cell strain, do not work with earlier versions of the 3 origin plasmids.<br/> | The pSB3K3 amplification failed. The bands indicated a much lower length than expected. After doing an alignment in Geneious, it appears our pSB3N5 primers we ordered for pSB3C5, which we are no longer using because of our competent cell strain, do not work with earlier versions of the 3 origin plasmids.<br/> | ||
| + | |||
Both the negative control and experimental of PCR purified Gisbson assembly from yesterday of pNT003 had 0 colonies. The transformation with 1 ul pUC 19 had no colonies.<br/> | Both the negative control and experimental of PCR purified Gisbson assembly from yesterday of pNT003 had 0 colonies. The transformation with 1 ul pUC 19 had no colonies.<br/> | ||
| + | |||
<table border="1"> | <table border="1"> | ||
<tr> | <tr> | ||
| Line 160: | Line 166: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| + | |||
BPA solutions fail to form uniform layers when poured on top of agar, but form a uniform layer when poured directly onto a clean plate. | BPA solutions fail to form uniform layers when poured on top of agar, but form a uniform layer when poured directly onto a clean plate. | ||
| + | |||
| + | |||
| + | <gallery> | ||
| + | File:BPA_on_agar.jpg|BPA on agar | ||
| + | File:BPA_clean_plate.jpg|BPA on clean plate | ||
| + | </gallery> | ||
| + | |||
| + | |||
==July 28== | ==July 28== | ||
Plate packaged fosmids to obtain titer <br/> | Plate packaged fosmids to obtain titer <br/> | ||
Revision as of 21:12, 29 July 2011
|
Project |
July 24Started overnight cultures of pSB3K3 July 25PCR'd parts for pNT002: R0040, K123001, B0014, pSB4A5 ResultsDecided not to use the pNT003 PCR'd insert, as the band of the correct length was much fainter than a lower length band. Chose not to do Gibson assembly of the positive GFP control, despite not having any green colonies last week, instead focusing on assembling pNT002 and pNT003. PCR concentrations
PSB3K3 miniprep concentrations
July 26Packaging of 9mix and (10mix + 10-2) ligations Results
The gel gave no bands in any lane, with or without the restriction digest. pNT003 + without the Spe1 digest had a faint smear, the other lanes had nothing. Chose to transform anyway All enrichment cultures but one showed growth on LB.
July 27Grew up cells for titering of packaged fosmids; reached OD600 of 0.974 ResultsThe 16s PCR from yesterday, shown in the gel today, had a band in the first lane of negative controls, indicating contamination. The pSB3K3 amplification failed. The bands indicated a much lower length than expected. After doing an alignment in Geneious, it appears our pSB3N5 primers we ordered for pSB3C5, which we are no longer using because of our competent cell strain, do not work with earlier versions of the 3 origin plasmids. Both the negative control and experimental of PCR purified Gisbson assembly from yesterday of pNT003 had 0 colonies. The transformation with 1 ul pUC 19 had no colonies.
July 28Plate packaged fosmids to obtain titer ResultsYesterday's 16s PCR failed, as there were no bands in control or experimental lanes. The vector was amplified and was the correct length. We redid it, but again there were no bands in the control or experimental lanes. Gel extraction of pNT003 insert was not much better than yesterday. Here are the concentrations:
|
 "
"