Team:Calgary/Project/Reporter
From 2011.igem.org
Emily Hicks (Talk | contribs) |
Emily Hicks (Talk | contribs) |
||
| Line 45: | Line 45: | ||
For preliminary data, <a href="https://2011.igem.org/Team:Calgary/Project/Preliminary_Data">click here</a>. | For preliminary data, <a href="https://2011.igem.org/Team:Calgary/Project/Preliminary_Data">click here</a>. | ||
<br> | <br> | ||
| - | For characterization | + | For characterization data, <a href="https://2011.igem.org/Team:Calgary/Project/Promoter/Final_Data">click here</a>. |
<br> | <br> | ||
| + | For electrochemical optimization, <a href="https://2011.igem.org/Team:Calgary/Project/Reporter/Optimization">click here</a><br> | ||
<style> | <style> | ||
#titlebar{margin-top: -6px;} | #titlebar{margin-top: -6px;} | ||
Revision as of 23:18, 28 October 2011









Reporter

What is a Reporter?
Being able to detect a particle in laboratory conditions can vary drastically from conditions in situ. After the presence of naphthenic acids is confirmed, we will require a reporter system for our bacteria to report their concentration accurately and with high resolution. Over the course of the project, Team Calgary considered three different reporter systems - colorimetric, fluorescent, and electrochemical. After weighing the advantages and disadvantages of each approach, we decided to use the electrochemical approach.
Why Electrochemical?
Oilsands tailings ponds samples are difficult to work with for several different reasons. First of all, tailings ponds samples are murky, and can have greatly varying compositions from pond to pond. It would be difficult to obtain accurate colorimetric data from these samples, and it would be similarly difficult to readily observe fluorescence. Because an electrochemical response does not rely on being able to see anything, Team Calgary determined the electrochemical reporter to be ideal for this purpose.
Electrochemical reporters offer several advantages over colorimetric and flourometric reporters: the signal is robust enough to not be altered by the presence of pollutants or sample turbidity; the oxidation potential of our analyte is distinct and can be readily distinguished between our desired signal and background noise; and the data is immediately available for analysis.
A Novel Application for the lacZ Gene
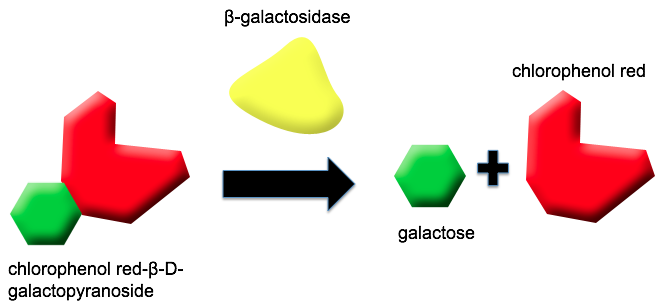
We decided to use the lacZ gene in our electrochemical reporter. Although traditionally, the lacZ gene (BBa_I732005) is used as a reporter for colorimetric assays (cleaving X-gal to create a blue pigment), the gene product ß-galactosidase, is capable of cleaving a variety of substrates. It has been shown by Biran et al. (1999) that ß-galactosidase can cleave p-aminophenyl-ß-D-galactopyranoside (PAPG), producing p-aminophenol (PAP). PAP can be oxidized at an electrode enabling detection of an electrochemical signal. It’s been shown that chlorophenolred-ß-D-galactopyranoside (CPRG) can also be cleaved by beta-galactosidase, producing chlorophenol red (CPR) and galactose. This substrate produces both a distinct color change (yellow to purple), and an electrochemical signal.
Building our System
One of the main reasons we selected a primarily electrochemical response for our system was that the response can be detected and processed digitally, without the need for subjective qualitative observations. The CPR analyte has an oxidation potential that is distinct from the tailings pond environment making it distinguishable from the background.
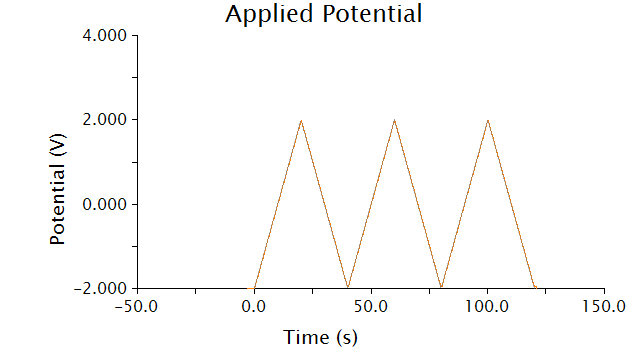
The voltammetric technique we used to detect chlorophenol red was cyclic voltammetry - the input voltage is shown below.
As voltage slowly increases and decreases, each species present in solution will undergo a redox reaction at its respective oxidation potential. Because we know the oxidation potential of our analyte, chlorophenol red (CPR), we can ignore any background noise or pollution using this method.
A three-electrode setup is required to perform cyclic voltammetry. The electrical current passes between the working and counter electrodes - and the potential difference between the reference electrode and counter electrode is controlled. This setup allows us to take voltage and current readings that do not interfere with each other.
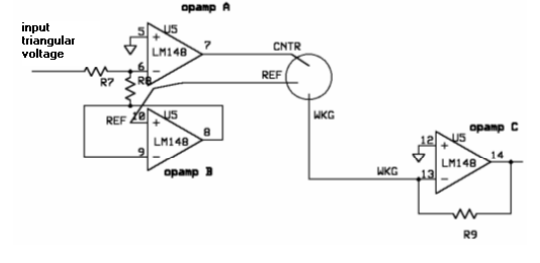
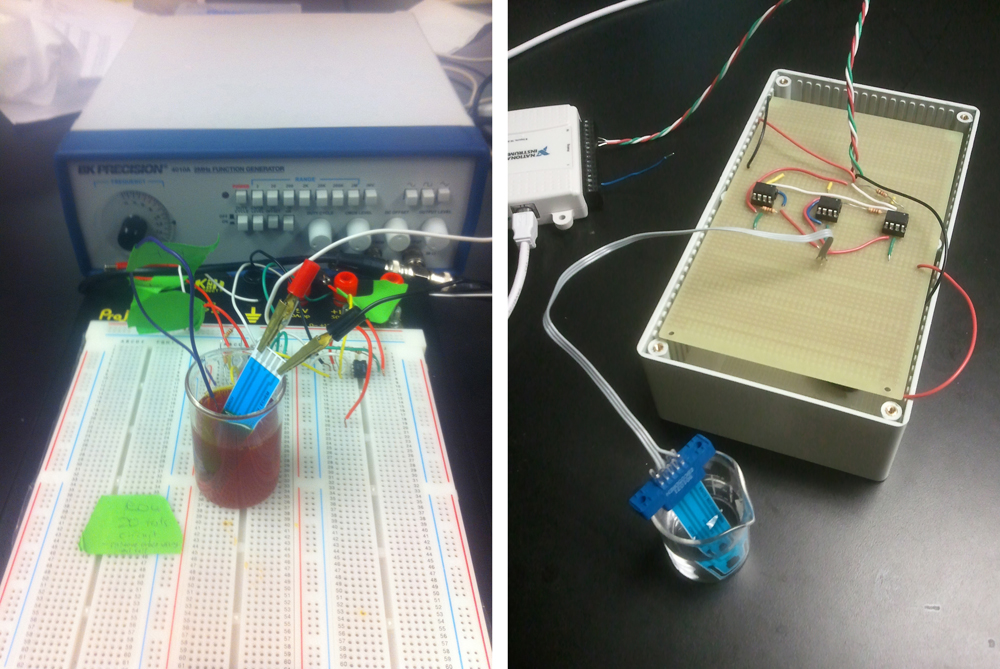
In order to perform cyclic voltammetry, specialized equipment is usually required. Such equipment is rare in biology labs, so we have created potentiostat prototypes - a device that outputs a known voltage and measures a corresponding current across two electrodes. These prototypes are cheap to build and have been shown to function as expected. However, for our measurements we used a commercial potentiostat and it's accompanying software to reduce the amount of time needed for data analysis.
For preliminary data, click here.For characterization data, click here.
For electrochemical optimization, click here

 "
"