Team:Kyoto/Digestion
From 2011.igem.org
(→Result) |
(→Discussion) |
||
| Line 80: | Line 80: | ||
=='''Discussion'''== | =='''Discussion'''== | ||
| + | |||
| + | '''Remaining cells and cell growth have no influence to the reducing sugar.'''<br> | ||
| + | |||
| + | |||
| + | '''There is some components that reaction with DNS.'''<br> | ||
<html><a href="/Team:Kyoto/Digestion/Modeling">Modeling</a></html><br> | <html><a href="/Team:Kyoto/Digestion/Modeling">Modeling</a></html><br> | ||
| - | + | ||
Revision as of 22:43, 5 October 2011
Contents |
Project Digestion
Introduction
An insect body is covered with a hard integument containing mainly chitin. To decompose the integument, we used ChiA gene, which encode chitinase. In order to measure the chitinase activity of the culture supernatant, we evaluated the effects of the medium and cell growth.
Streptomyces is a kind of prokaryotic bacteria which decompose bodies in the nature. We extract chitinase gene from this bacterium and introduce into Escherichia coli. The secretion-signal sequences are included in this gene so that the protein coded by them will go out without occurring cell lysis.
After assembling all genes, we examined the activity of this enzyme in quantitative way, and seek equations of production of chitinase and N-acetylglucosamin.
Method
Construction
We created following construction to allow secretion of chitinase, chiA1. This gene is regulated by strong lactose promoter, BBa_R0011. We used Streptmyces’s RBS into this constructions, because reference article [1] used that to allow E.coli to secrete the protein.
要変更→
Assay
We performed 3,5-Dinitrosalicylic acid assay (DNS assay), because this assay takes a little time, costs a little money and was used in the previous article measuring chitinase activities [1]. DNS assay is based on this fact: 3,5-dinitorosalicylic acid (DNS), whose color is yellow, reacts with reducing saccharide by boiling and changes into 3-amino-5-nitorosalicylic acid, whose color is brawn.
The more the amount of reducing sugar is, the more this coloring reaction proceeds. We can quantitatively evaluate the degree of this coloring reaction by measuring OD 550, because OD 550 is in direct proportion to the amount of reducing sugar.
We will react DNS reagent with the media where chitin and E.coli introduced chitinase gene are added. If chitinase is secreted in media, chitin is decomposed into reducing sugar, for example, N-acetylglucosamin and coloring reaction proceeds. Therefore, by measuring OD 550, we can indirectly measure chitinase activities.
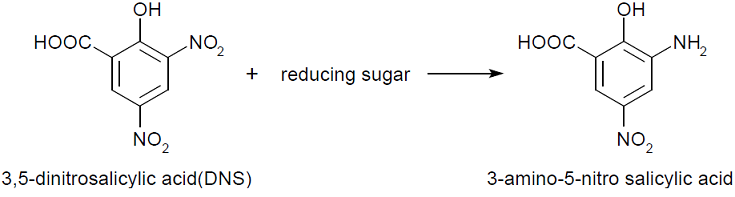
Result
This figure shows the overall of DNS assay
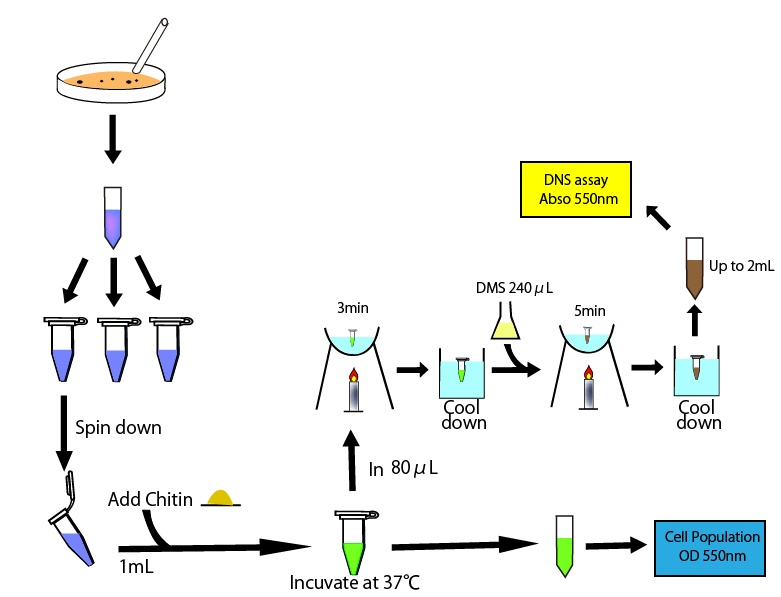
To measure chitinase activity by DNS assay, we thought to need four following things to get accurate result. We need
- to plot the relationship of reducing sugar concentration and OD 550
- to evaluate the impact of components of media on OD 550
- to evaluate the impact of remeineded E.coli in media on OD 550
- to measure chitinase activity
1. Standard Measurement for ChiA1.
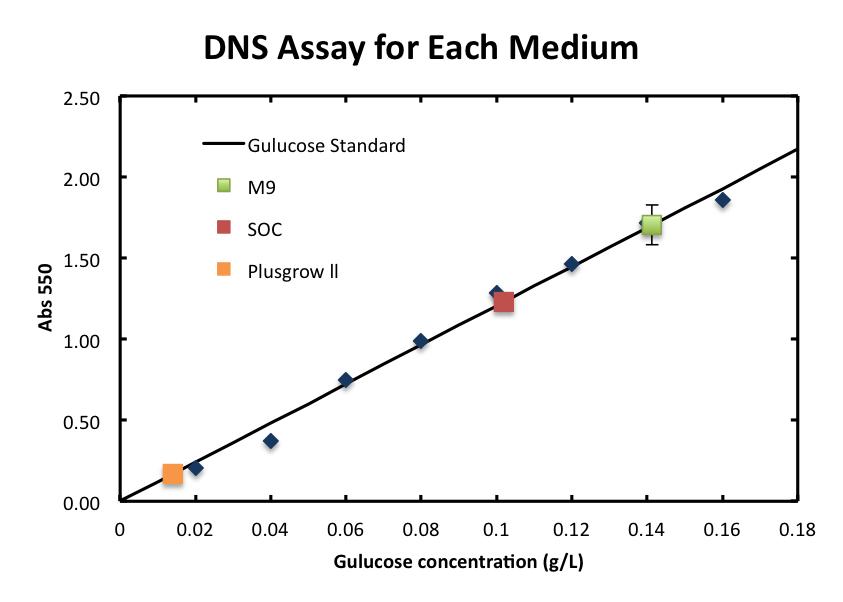
In order to measure the product of the enzyme reaction, N-acetylglucosamin, quantitatively, we prepared the standard curve using glucose as standard. It is known that the correlation between glucose concentration and the absorbance can be plotted in linaer.
- From the result, a strong correlation between glucose concentration and its A550 was observed.
2. Consideration of medium and growth of E.coli.
To get accurate values of absorbance, it is needed to consider the remained cells in supernatant. Since we incubate the supernatant for hours for the enzyme reaction, cells can grow, produce chitinase and consume glucose, N-acetylglucosamin, etc; this can influence to DNS assay.
2-1 The absorbance of supernatant itself.
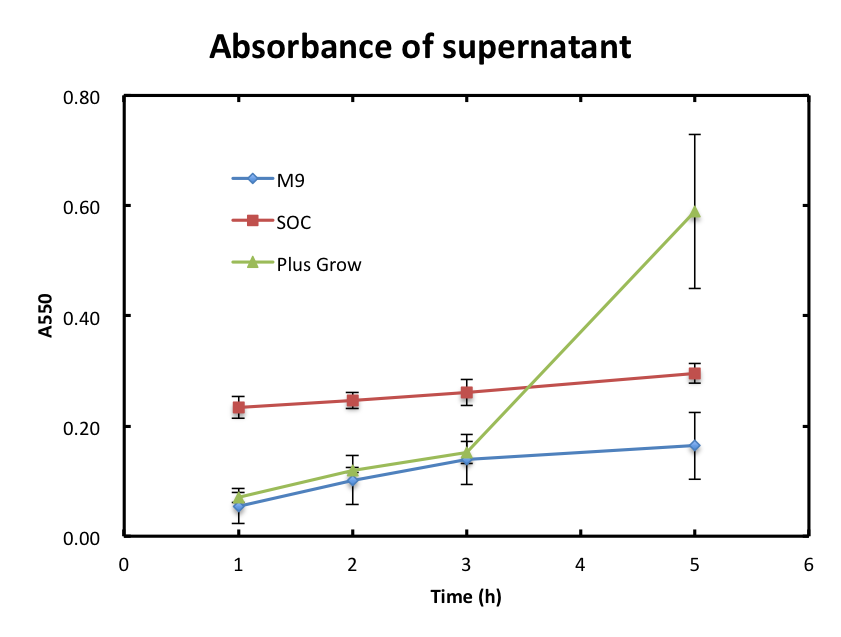
- In this experiment, we checked if cells were still observed in the supernatant and assayed the growth behavior of cells. Figure 2 shows the A550 of supernatant with time. The absorbance values of M9 were increased slowly and they were smaller than 0.2 during the incubation. The absorbance values of SOC were also increased slowly and they were higher than those of M9. The absorbance of values of Plusgrow Ⅱ were increased obviously between the 3h and 5h.
2-2 The DNS assay of supernatant.
- From figure 2, the increase in absorbance of Plusgrow Ⅱ was thought to be due to the cell growth. So, we considered that cells still existed in M9 and SOC medium though the significant cell growth couldn't observed.
- In this experiment, we evaluated the influence of cells and its growth to the DNS assay. In other words, we confirmed if cells consume glucose and affect the DNS assay. Figure 3 shows the absorbance of each culture supernatant. The values of M9 and Plusgrow Ⅱ were almost zero during the incubation. The absorbance of SOC was observed, but there was little change during the incubation.
From figure 2 and 3, the cell growth didn't affect to the absorbance of supernatant. About M9 and SOC medium, each medium contains only glucose as sugar source and it is considered that glucose was mostly consumed after overnight culture and this is the reason that cells growth didn't observed and the absorbance values of DNS assay were almost zero.
2-3 The DNS assay of medium.
- We checked the influence of each medium to the DNS assay. Figure 4 shows the background absorbance of each medium. The absorbance of M9 was 1.7±0.1, SOC was1.227±0.007, and Plusgrow Ⅱ was 0.17±0.02.
Discussion
Remaining cells and cell growth have no influence to the reducing sugar.
There is some components that reaction with DNS.
The results of this measurement and the fig 1 graph enabled us to calculate the amount of digested chitin, showing the relative activity of chitinase.
To overcome these barriers, we decided detail plan of our assay. From the result fig 3, SOC medium cultured E.coli overnight would still include too much amount of reducing materials and, from fig 3. plas-grow enabled reminded E.coli to grow rapidly. However, as for M9 medium, the increase of absorbance was lowest and there were fewest E.coli after 5 hour culture. So we choose M9 as the medium we will used in our assay. Another barrier, interruption of remained E.coli, can be avoided by the following way, conducting DNS assay before chitin is applied and after chitin is decomposed. The difference of these two assays’ data will show the real activity of chitinase.
Reference
[1] “Actinobacteria.” Internet: http://en.wikipedia.org/wiki/Actinobacteria [Nov. 5, 2011]
[2] H. Ikeda, J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, S. Omura, “Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis.” Nat Biotechnol., vol. 21, no. 5 pp. 526-31, Apr. 2003
[3] S. Omura, H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, “Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites.” Proc Natl Acad Sci U S A. vol. 98, no. 21 pp. 12215-20, Oct. 9
[4] H. Sakuzou, ”還元糖の定量法(生物化学実験法)” Kyoto University: Japan Scientific Soceties Press
[5] S. Kongruang, M. J. Han, C. I. Breton, M. H. Penner, “Quantitative Analysis of Cellulose-Reducing Ends.” Appl Biochem Biotechnol. Vol. 113, no. 116 pp. 213-31, Spring 2004
 "
"