Team:HKU-Hong Kong/Parts
From 2011.igem.org
| Line 3: | Line 3: | ||
|style="font-family: georgia, helvetica, arial, sans-serif;font-size:2em;color:#01DF01;"|Sample Data Page | |style="font-family: georgia, helvetica, arial, sans-serif;font-size:2em;color:#01DF01;"|Sample Data Page | ||
|- | |- | ||
| - | |style="width: | + | |style="width:900px;"| |
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| Line 16: | Line 16: | ||
|style="width:900px;"| | |style="width:900px;"| | ||
By expressing the tetR:H-NS fusion proteins, the tetR part of the proteins wound recognize and specifically bind to the tetR binding region (tetO2). There are two tet-R binding sites as tet-R form a dimer as in the tet repressor- operator system. As the fusion proteins bound to tet-O, it is expected that the H-NS part of the fusion protein would attract and oligomerize with other native H-NS proteins inside the cell. With the oligomerization of the fusion proteins and native H-NS, the DNA covered by the oligomers is expected to trap the RNA polymerase during transcription, thus gene (GFP) repression is achieved. | By expressing the tetR:H-NS fusion proteins, the tetR part of the proteins wound recognize and specifically bind to the tetR binding region (tetO2). There are two tet-R binding sites as tet-R form a dimer as in the tet repressor- operator system. As the fusion proteins bound to tet-O, it is expected that the H-NS part of the fusion protein would attract and oligomerize with other native H-NS proteins inside the cell. With the oligomerization of the fusion proteins and native H-NS, the DNA covered by the oligomers is expected to trap the RNA polymerase during transcription, thus gene (GFP) repression is achieved. | ||
| - | + | |- | |
| - | + | |style="width:900px;"| | |
| - | + | <div ALIGN=CENTER> | |
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Sample Data Table.png|650px]] | ||
| + | |- | ||
| + | |} | ||
| + | </div> | ||
| + | |} | ||
| + | {| style="width:900px;background:#000000;text-align:justify;font-family: georgia, helvetica, arial, sans-serif;color:#ffffff;margin-top:25px;" cellspacing="20" | ||
| + | |- | ||
| + | |style="width:900px;"| | ||
'''Experience:''' | '''Experience:''' | ||
Revision as of 16:34, 5 October 2011
| Sample Data Page |
|
By expressing the tetR:H-NS fusion proteins, the tetR part of the proteins wound recognize and specifically bind to the tetR binding region (tetO2). There are two tet-R binding sites as tet-R form a dimer as in the tet repressor- operator system. As the fusion proteins bound to tet-O, it is expected that the H-NS part of the fusion protein would attract and oligomerize with other native H-NS proteins inside the cell. With the oligomerization of the fusion proteins and native H-NS, the DNA covered by the oligomers is expected to trap the RNA polymerase during transcription, thus gene (GFP) repression is achieved. |
|
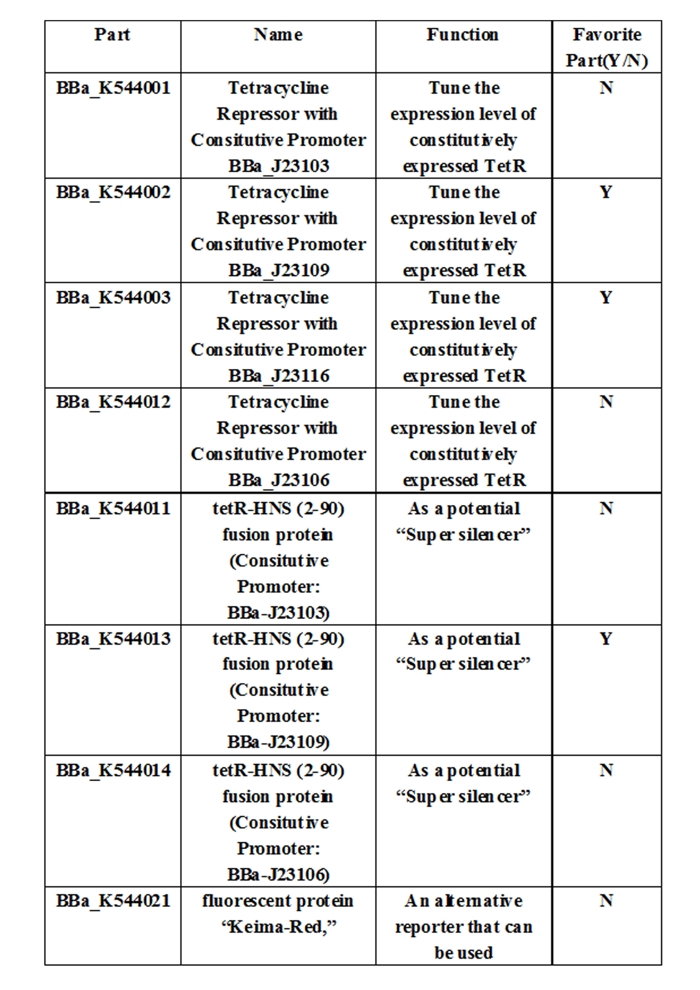
Experience: We have entered the confirmation data for the characterization of the selected constitutive promoters we have used. Of the 4 promoters we have used in the project, our team considered that J23109 or J23116 are more suitable promoters in creating the super silencer repression system. These parts are:
|
 "
"