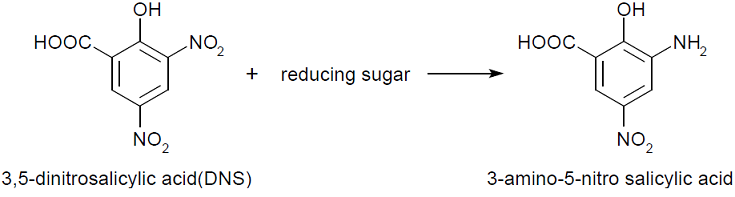Team:Kyoto/Digestion
From 2011.igem.org
(→Reference) |
Tomohiro:n (Talk | contribs) (→Discussion) |
||
| Line 63: | Line 63: | ||
== '''Discussion''' == | == '''Discussion''' == | ||
| + | From result of preliminary experiments, we found several problems. | ||
| + | ・the affection of medium itself | ||
| + | |||
| + | The components of each medium also reduced 3,5-dinitorosalicylic acid and would cause error in the assay. | ||
| + | |||
| + | ・interruption of remaining E.coli. | ||
| + | |||
| + | Even though we use the centrifugal supernatant, there was still some E.coli. we found they could interrupt data because they would decompose reducing sugers. | ||
| + | |||
| + | To overcome these barriers, we decided detail plan of our assay. | ||
| + | |||
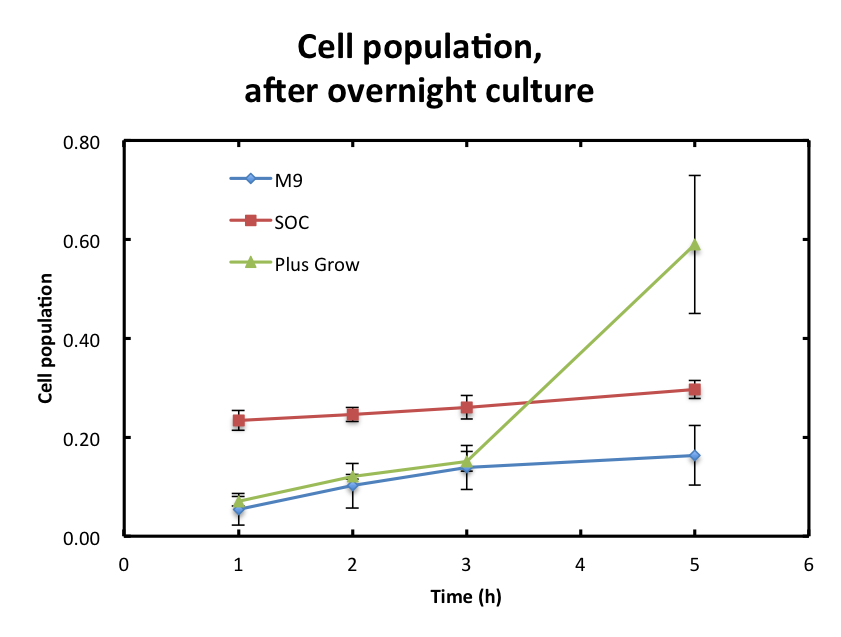
| + | From the result fig 3, SOC medium cultured E.coli overnight would still include too much amount of reducing materials and, from fig 3. plas-grow enabled reminded E.coli to grow rapidly. However, as for M9 medium, | ||
== '''Reference''' == | == '''Reference''' == | ||
Revision as of 17:18, 4 October 2011
Contents |
Project Digestion
Introduction
Streptomyces is a kind of prokaryotic bacteria which decompose bodies in nature[1]. We extract protease and chitinase genes from this bacterium and introduce into Escherichia coli. Secretion-signal sequences are included in these genes so that the proteins coded by them will go out without occurring cell lysis. After assembling all genes, we examined the activity of these two enzymes in both of and quantitative ways.
Method
Construction
We created following constructions to allow secretion of Serine protease, SAM-P20 and chitinase, chiA1. These genes are regulated by lactose promoter, BBa_R0011. We used Streptmyces’s RBS into these constructions, because reference article [1] used them to allow E.coli to secrete these proteins.
Assay
We performed following qualitative and quantitative assays. These method take a little time and cost a little money.
Serine Protease
- Experiment 1 : Skim milk-hydroryzing assay
- In order to identify the expression of SAM-P20 gene, a skim milk-hydroryzing assay was performed. A plate containing 2% skim milk, IPTG(final concentration, 0.5mM), and 0.1% yeast extract was used for this assay.
- Experiment 2 : Measurement of enzyme activity
- In order to measure the activity of serine protease, the fluorescent assay was done, using fluorescein-labeled casein as a substrate.
Chitinase A
- Experiment 1 : Chitin plate assay
- In order to identify the expression of ChiA genes, Chitin plate assay was conducted. We used a LB plate whose surface chitin flour was on
- Experiment 2 : 3,5-Dinitrosalicylic acid assay (DNS method)
- This assay is based on this fact: 3,5-dinitorosalicylic acid (DNS) is changed into 3-amino- 5-nitorosalicylic acid by reducing saccharide in reaction solution and the absorbance of this liquid increase in direct proportion to the amount of reducing sugar.
- If chitinase is secreted, chitin in media was decomposed to, for example, N-acetylglucosamin,that is ,reducing sugar. Therefore, The absorbance of the media added DNS will increase.
Result
Chitinase A1
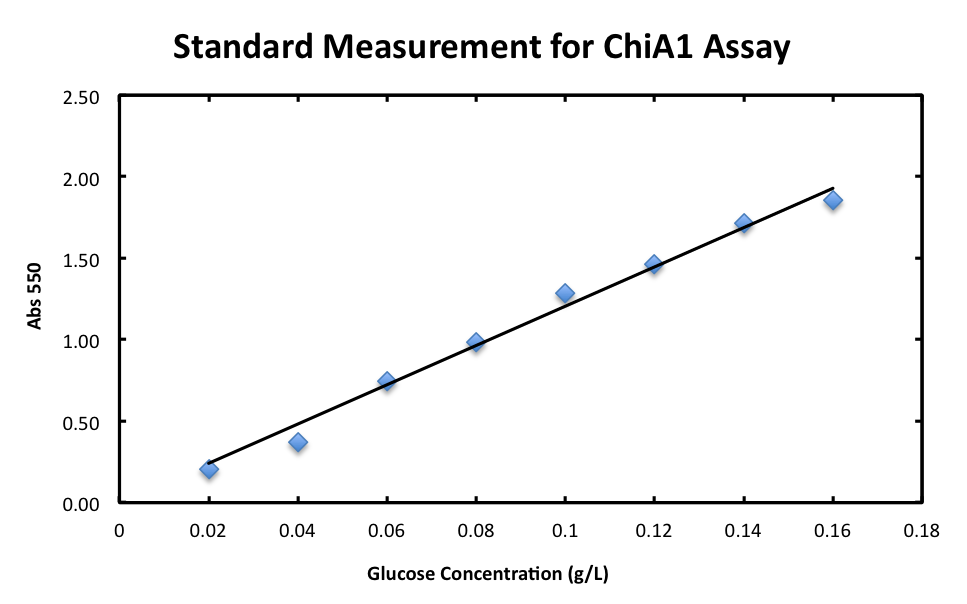
1. Standard Measurement for ChiA1.
- From the result, a strong correlation between glucose concentration and its A550 was observed.
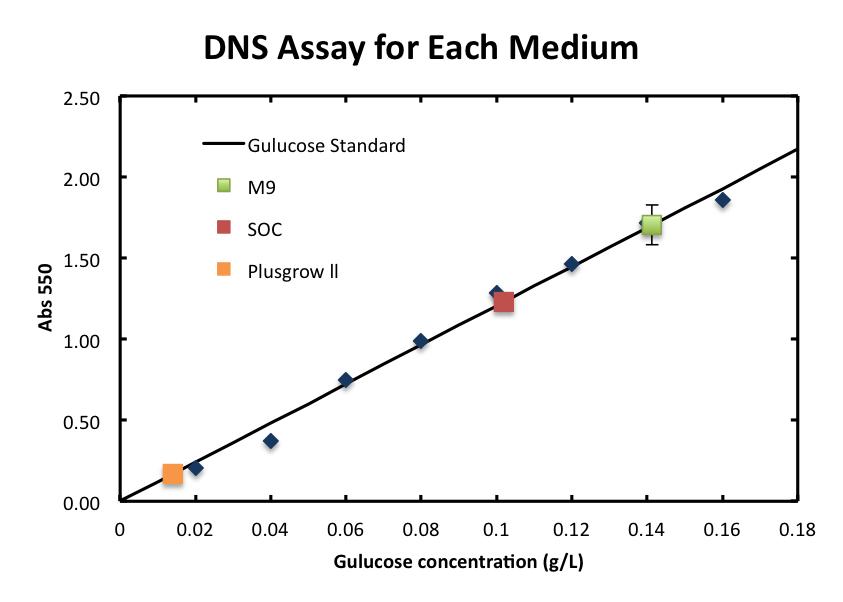
2. Consideration of medium and growth of E.coli.
We checked the influence of each medium to the DNS assay. Figure 2 shows the background absorbance of each medium. The absorbance of M9 was 1.7±0.1, SOC was, and Plusgrow Ⅱ was 0.17±0.02.
Discussion
From result of preliminary experiments, we found several problems.
・the affection of medium itself
The components of each medium also reduced 3,5-dinitorosalicylic acid and would cause error in the assay.
・interruption of remaining E.coli.
Even though we use the centrifugal supernatant, there was still some E.coli. we found they could interrupt data because they would decompose reducing sugers.
To overcome these barriers, we decided detail plan of our assay.
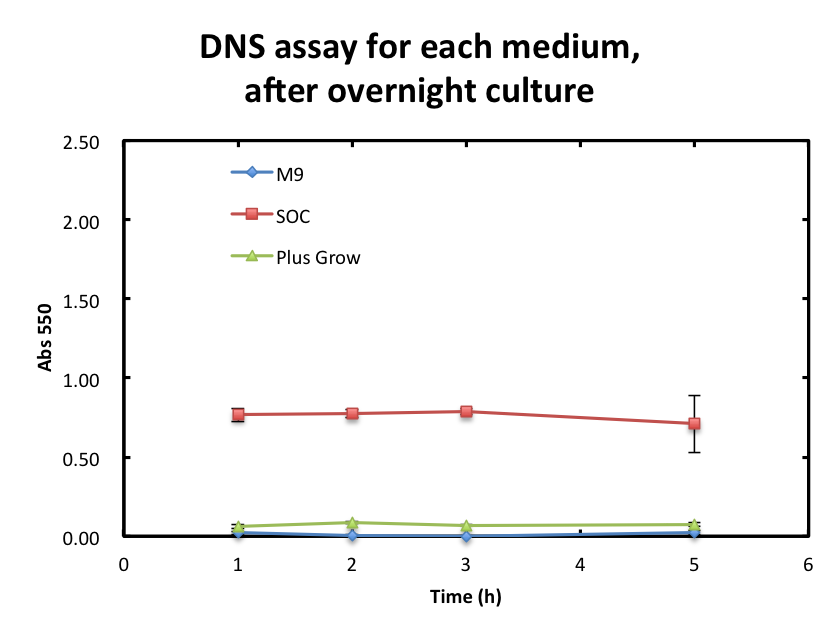
From the result fig 3, SOC medium cultured E.coli overnight would still include too much amount of reducing materials and, from fig 3. plas-grow enabled reminded E.coli to grow rapidly. However, as for M9 medium,
Reference
[1] “Actinobacteria.” Internet: http://en.wikipedia.org/wiki/Actinobacteria [Nov. 5, 2011]
[2] A. S. Khalil, J. J. Collins, “Synthetic biology: applications come of age.” Nat Rev Genet., vol. 11, no. 5 pp. 367-79, May. 2010.
[3] H. Ikeda, J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, S. Omura, “Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis.” Nat Biotechnol., vol. 21, no. 5 pp. 526-31, Apr. 2003
[4] S. Omura, H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, “Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites.” Proc Natl Acad Sci U S A. vol. 98, no. 21 pp. 12215-20, Oct. 9
[5] H. Sakuzou, ”還元糖の定量法(生物化学実験法)” Kyoto University: Japan Scientific Soceties Press
[6] S. Kongruang, M. J. Han, C. I. Breton, M. H. Penner, “Quantitative Analysis of Cellulose-Reducing Ends.” Appl Biochem Biotechnol. Vol. 113, no. 116 pp. 213-31, Spring 2004
 "
"