Team:UNICAMP-EMSE Brazil/Results
From 2011.igem.org
Neshich.iap (Talk | contribs) (→Device 3 testing, Protein Secretion System) |
Neshich.iap (Talk | contribs) (→Device 3 testing, Protein Secretion System) |
||
| Line 64: | Line 64: | ||
==Device 3 testing, Protein Secretion System== | ==Device 3 testing, Protein Secretion System== | ||
| - | The assembled devices related to secretion system (Hemolysin secretion system under control of SoxS – SoxS-HlyB-HlyD-TolC) was tested under laboratory conditions using GFP as reporter. We were able to validate the parts HlyB ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554007 BBa_K554007]), HlyD ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554008 BBa_K554008]), TolC ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554009 BBa_K554009]) and SoxS HlyB HlyD TolC device ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554013 K554013]). | + | The assembled devices related to secretion system (Hemolysin secretion system under control of SoxS – SoxS-HlyB-HlyD-TolC) was tested under laboratory conditions using GFP as reporter. We were able to validate the parts HlyB ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554007 BBa_K554007]), HlyD ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554008 BBa_K554008]), TolC ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554009 BBa_K554009]), HlyA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554002 BBa_K554002] and SoxS HlyB HlyD TolC device ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554013 K554013]). |
[[Image:UNICAMP_EMSE_secretion_device_schema2.jpg|center|730px]] | [[Image:UNICAMP_EMSE_secretion_device_schema2.jpg|center|730px]] | ||
Revision as of 03:12, 29 September 2011

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |

Contents |
Overview
Functional tests
The students assembled the devices subparts: the SoxR/SoxS sensor (Device 2, NO sensor device/ IL-10 producer), the Adrenaline sensor (Device 1, CA/AI-3 sensor device/ IL-12 producer), and the hemolysin secretion system (Device 3, Protein Secretion System).
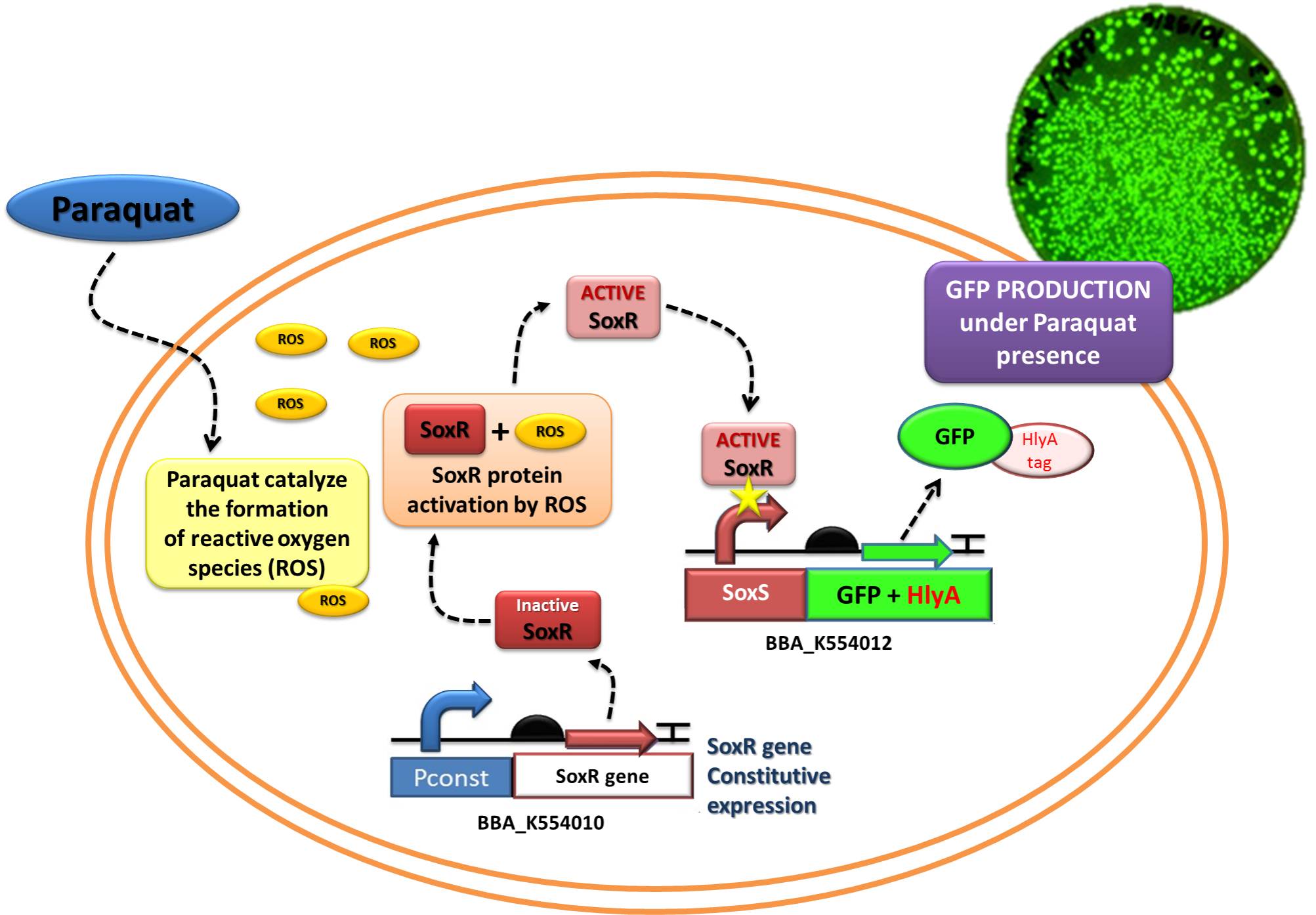
The assembled devices related to oxidative-stress sensing ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554010 SoxR transcription factor under control of a strong constitutive promoter - BBa_J23119]) and effector function in response to oxidative stress ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554012 a generic protein fusioned with the C-terminal tail of Hemolysin A - HlyA -under control of the SoxS promoter, recognized by the SoxR transcription factor]) were tested under laboratory conditions using GFP as reporter.
Device 2 testing: SoxR/SoxS system regulating GFP production
The Device 2 is a modification of Stanford team anti-inflammatory device (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), which comprises SoxR gene (BBa_K223047) under control of a Constitutive Promoter (BBa_J23119) and SoxS promoter (BBa_K223001 – deleted part), coupled to human IL-10 gene (sequence designed by the team, improved for bacterial expression).
In order to test the ability of Jedi Bacteria in sensing NO levels and activating genes linked to SoxS promoter, we built a Device testing, with GFP linked to SoxS promoter, as it is shown in the following schema (Figure 1):
Methods
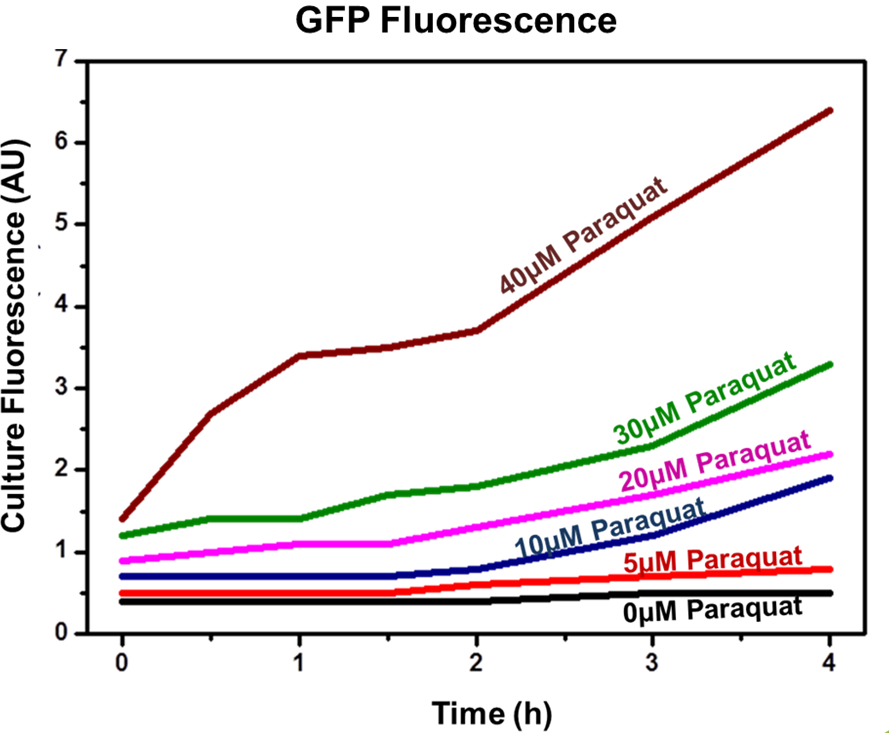
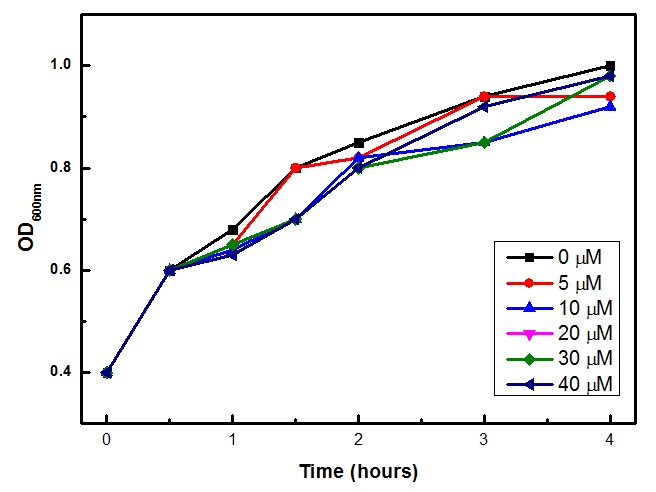
Competent E. coli DH5α strain cells were transformed with a pSB1A2 vector (Ampicillin resistant) carrying both the sensor (Strong_Constitutive_promoter + RBS + SoxR + Terminator) and the effector (SoxS_pormoter + RBS + GFP + HlyA + Terminator) devices using a chemical shock protocol. Transformed bacteria were plated on solid LB medium with 50 μg/mL Ampicillin and grown at 37ºC overnight. Surviving colonies were grown on liquid LB medium containing 50 μg/mL Ampicillin. Oxidative stress was induced by adding increasing concentrations of http://en.wikipedia.org/wiki/Paraquat Paraquat (Methyl viologen dichloride hydrate - Sigma), an oxidative stress inducer in bacteria. Final Paraquat concentrations were: 0 μM (control), 5 μM, 10 μM, 20 μM, 30 μM and 40 μM. Induction of the designed sensor/effector mechanism was accessed by fluorescence using a fluorometer (SLM – Aminco; 4 nm bandpass and 10 mm) with excitation in 500 nm and emission spectra from 508-550 nm. In order to access if increasing concentrations of Paraquat could inhibit culture growth due to it´s toxicity, optical density (OD) levels of the culture were measured during the incubation time with Paraquat. The ability to recognize NO (nitric oxide), an inflammation signal molecule, was characterized for SoxR/SoxS sensor, and found to be FUNCTIONAL. In the section below we present the detailed results.
Results
Achieved results indicated that the designed sensor/effector device system was capable of inducible production of GFP (or another generic protein controlled by the sensor). In addition, protein induction can be modulated through varying inducer concentration (Figure 2). Higher concentrations of Paraquat exhibited higher fluorescence levels, which indicates increased GFP concentrations. No plateau was achieved using the highest tested concentrations.
The experimental concentrations of Paraquat did not show significant differences in cell growth as shown by OD levels in Figure 3.
Discussion
The SoxR/SoxS system is one of the best characterized redox-sensing mechanisms in bacteria. Under oxidative stress conditions, activation of this system results in a cascade effect that ends in the activation of more than 16 genes that can counteract the harmful effect of superoxide and other oxidative radicals (Pomposiello and Demple 2001).
This device is a modification of Stanford team anti-inflammatory device (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), which comprises SoxR gene (BBa_K223047) under control of a Constitutive Promoter (BBa_J23119) and SoxS promoter (BBa_K223001 – deleted part). Although this system has already been designed and used in previously iGEM competitions (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), we took advantage of the availability of synthesized sequences to design new parts (SoxR and SoxS) that conforms the common biobrick standards in the iGEM registry. Our team has improved the parts, designing them according to the assembly standard 23, which allows the insertion of more than one coding region in frame. In addition, the new parts are in transcription unit format (with RBS).
These newly designed parts were capable of sensing different concentrations of the inducer, resulting in an increased production of the protein under control of the SoxS promoter (in this case, GFP).
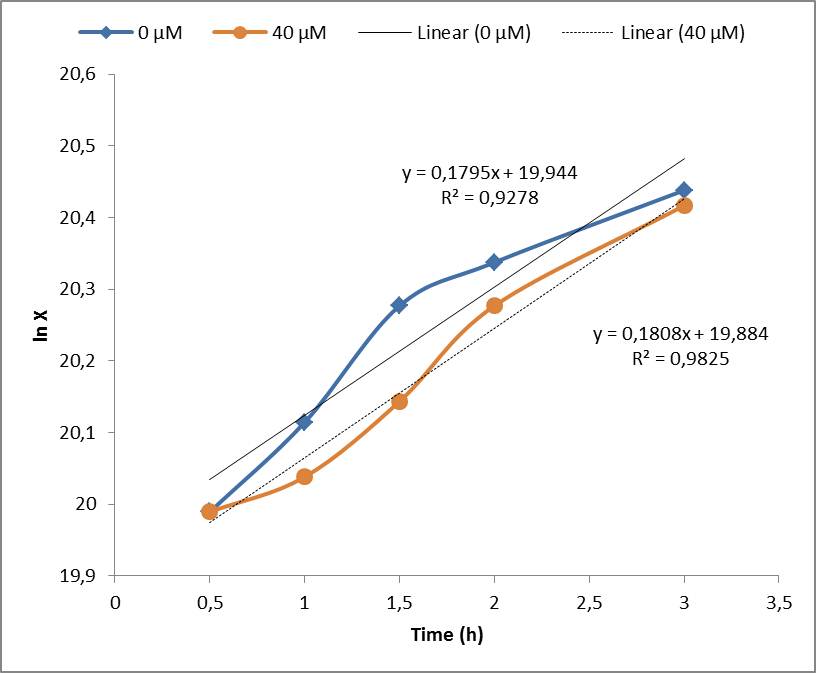
Under the experimental conditions, no cell growth inhibition was observed and confirmed by the calculations of the specific growth rate (μ). No significant difference was found in control experiment (0 μM of Paraquat) and cell induced with 40 μM of Paraquat, both presented μ= 0,18 h-1. Although the Stanford 2009 team showed that Paraquat concentrations between 60 μM and 80 μM can inhibit E. coli cell growth due to enhanced toxicity, but these concentrations were not included in our tests.
Device 3 testing, Protein Secretion System
The assembled devices related to secretion system (Hemolysin secretion system under control of SoxS – SoxS-HlyB-HlyD-TolC) was tested under laboratory conditions using GFP as reporter. We were able to validate the parts HlyB ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554007 BBa_K554007]), HlyD ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554008 BBa_K554008]), TolC ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554009 BBa_K554009]), HlyA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554002 BBa_K554002] and SoxS HlyB HlyD TolC device ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K554013 K554013]).
Methods
To access GFP secretion, competent E. coli DH5α strain cells were transformed simultaneously with a pSB1C3 vector (Chloramphenicol resistant) carrying both the sensor (Strong_Constitutive_promoter + RBS + SoxR + Terminator) and the effector (SoxS_promoter + RBS + GFP + HlyA + Terminator), and pSB1AK3 (Ampicillin resistant) carrying the secretion system (SoxS_promoter + RBS + HlyB + HlyD + TolC + Terminator). As a non-secretion control, E. coli harboring only the pSB1C3 vector with both sensor and effector systems was used.
Oxidative stress was induced by adding increasing concentrations of Paraquat (Methyl viologen dichloride hydrate - Sigma), an oxidative stress inducer in bacteria. The secretion was tested in cultures harboring A) and B) (Figure ) using 0 μM and 40 μM of Paraquat as described above. After 3 hours samples were collected, centrifuged (4000 rpm / 10 min; to avoid cell lysis) and the supernatant was collected and centrifuged again (13000 rpm / 10 min; to remove remaining cells). The supernatant fluorescence was measured in fluorometer (SLM – Aminco; 4 nm bandpass and 10 mm) with excitation in 500 nm and emission spectra from 508-550 nm. The GFP fluorescence was also detected in cells by fluorescence microscopy (Olympus).
Results
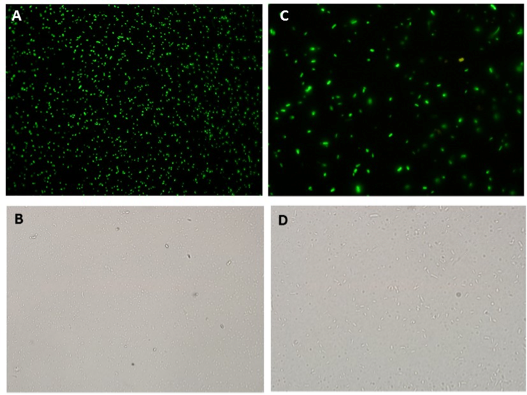
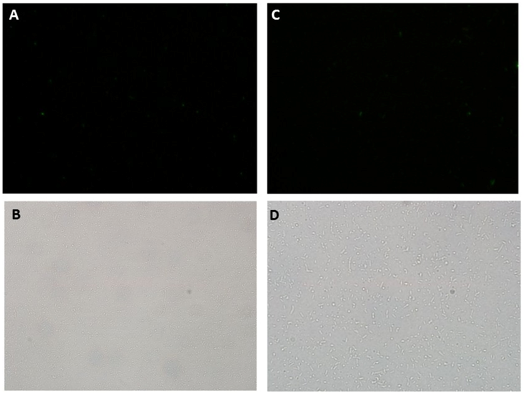
Microscopy data revealed GFP expression in the Paraquat induced cultures (Figure 2) but not in the non-induced one (Figure 3).
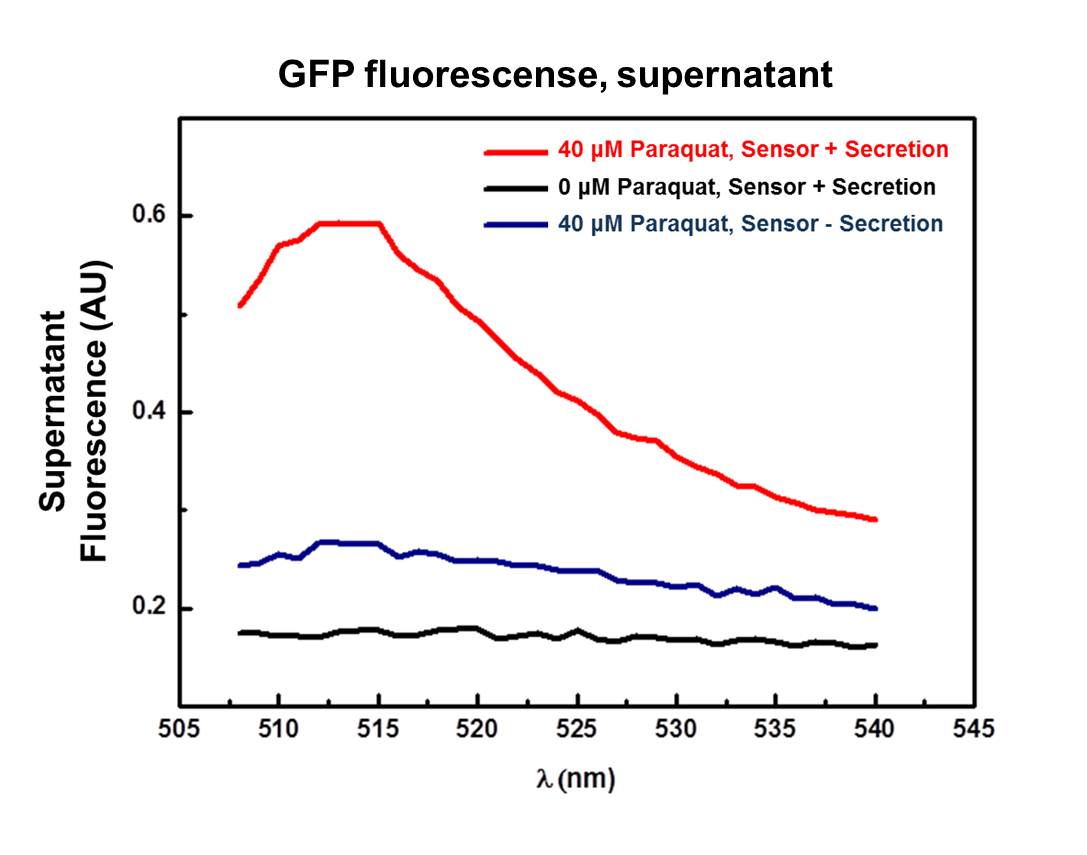
GFP secretion was confirmed by fluorescence emission and estimated to be approximately 6% of total protein, according to fluorescence levels. Significant levels of GFP fluorescence were found only in the supernatant of Paraquat induced cultures containing both the sensor/effector and the secretion systems but not in the non-induced cultures and in the cultures containing only the sensor/effector system (Figure 4).
Discussion
This device is a modification of Stanford team 2009 secretion system composite [http://partsregistry.org/Part:BBa_K223062 BBa_K223062]. The secretion system was improved according to biobrick assembly standard 23, and modified by deletion of Hemolysin C (toxin modified enzyme) which is responsible for acylation and activation of HlyA protein (not HlyA secretion signal peptide, used in fusion to GFP, and IL’s). When HlyA protein is activated by HlyC enzyme, is secreted and can cause cytotoxic effects to many cell types. Thus HlyC is related only to pathogenesis and not secretion. This new part was able to secrete GFP fusioned to HlyA secretion peptide signal in response to Paraquat induction of the sensor/effector/secretion system, as showed by supernatant fluorescence.
References
Pomposiell P.J., Demple B. (2001). Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends in Biotechnology. 19(3):109-114. [http://www.ncbi.nlm.nih.gov/pubmed/11179804 Link to PubMed]
We would like to thank the following people for the support given in the testing experiments
- Msc. Viviane Cristina Heinzen da Silva, Laboratório de Bioquímica de Proteínas (LBqP) - IQ/Unicamp
- Msc. Gleidson Silva Teixeira, Laboratório de Genômica e Expressão (LGE) - IB/Unicamp
- Dr. Joan Grande Barau, Laboratório de Genômica e Expressão (LGE) - IB/Unicamp

 "
"