Team:MIT/Results/
From 2011.igem.org
| Line 204: | Line 204: | ||
<li><a href="#gal4">Gal4/UAS promoter</a></li> | <li><a href="#gal4">Gal4/UAS promoter</a></li> | ||
<li><a href="#laci">LacI/Hef1a-LacO repressor</a></li> | <li><a href="#laci">LacI/Hef1a-LacO repressor</a></li> | ||
| - | <li><b><a href="#cascade">Gal4→LacI¬rtTA3→ | + | <li><b><a href="#cascade">Gal4→LacI¬rtTA3→2-input AND gate</a> </b></li> |
<li><a href="#cmv-to">CMV-TetO promoter</a></li> | <li><a href="#cmv-to">CMV-TetO promoter</a></li> | ||
<li><a href="#mnt">Mnt-VP16/Mnt promoter</a></li> | <li><a href="#mnt">Mnt-VP16/Mnt promoter</a></li> | ||
| Line 236: | Line 236: | ||
</p> | </p> | ||
| - | <h3><a id="cascade">Gal4-VP16→LacI¬rtTA3→ | + | <h3><a id="cascade">Gal4-VP16→LacI¬rtTA3→2-input AND gate</a></h3> |
<a href="http://s1087.photobucket.com/albums/j476/MITiGEM/?action=view&current=cvnqioeuwhvnioehf-1.jpg" target="_blank"><img src="http://i1087.photobucket.com/albums/j476/MITiGEM/cvnqioeuwhvnioehf-1.jpg" border="0" alt="Photobucket" style="width:100%"></a> | <a href="http://s1087.photobucket.com/albums/j476/MITiGEM/?action=view&current=cvnqioeuwhvnioehf-1.jpg" target="_blank"><img src="http://i1087.photobucket.com/albums/j476/MITiGEM/cvnqioeuwhvnioehf-1.jpg" border="0" alt="Photobucket" style="width:100%"></a> | ||
<img src='https://static.igem.org/mediawiki/igem.org/6/6d/Circuit_Diagram_Cascade_A.jpg' style="width:100%"> | <img src='https://static.igem.org/mediawiki/igem.org/6/6d/Circuit_Diagram_Cascade_A.jpg' style="width:100%"> | ||
<h4>Explanation:</h4> | <h4>Explanation:</h4> | ||
| - | <p>Here we show an experimental result of an example of what we call our internal processing module, which is essentially a | + | <p>Here we show an experimental result of an example of what we call our internal processing module, which is essentially a 2 input chemical AND gate. Taking the individual functional transcription factor systems characterized above, we combine them into an AND gate with tewo inputs: IPTG and doxycycline levels. The system integrates the UAS/GV16, rtTA3/TRE, and lacI/lacO systems described above. IPTG is required to relieve inhibition of rtTA3 expression. rtTA3 then needs doxycycline to activate TRE. Therefore, we require both IPTG and dox to achieve expression of our final Delta-mCherry reporter. |
| + | <p>The data show the desired 2 input AND behavior. We see maximum Delta-mCherry expression at the highest levels of both dox and IPTG. We also observe significant activation at high dox levels even in the absence of IPTG. This suggests some leakiness in the lacI inhibition, allowing for some rtTA3 to be produced and activated by dox./p> | ||
<p>The above result, obtained from flow cytometry by measuring the mCherry fluorescence of transfected Hek293 cells in the presence of different IPTG and doxycycline concentrations, are as we expected. In the absence of doxycycline, rtTA3 cannot activated the TRE promoter, and with increasing IPTG concentration, expressed LacI cannot inhibit the Hef1a-LacO promoter, and so we see a rise in Delta-mCherry due to this. | <p>The above result, obtained from flow cytometry by measuring the mCherry fluorescence of transfected Hek293 cells in the presence of different IPTG and doxycycline concentrations, are as we expected. In the absence of doxycycline, rtTA3 cannot activated the TRE promoter, and with increasing IPTG concentration, expressed LacI cannot inhibit the Hef1a-LacO promoter, and so we see a rise in Delta-mCherry due to this. | ||
Revision as of 03:25, 28 September 2011
DNA Delivery Systems and Data Collection
Transfection Using Lipofectamine 2000 (Invitrogen)
To introduce our engineered genetic parts into mammalian cells, we employed the Lipofectamine 2000 reagent from Invitrogen, and obtained at best an 80% transfection efficiency for Hek293 cells and 10% transfection efficiency for CHO cells.Figure 1: Hek293 Lipofectamine Transfection Results
A

B

C

(1A) This scatter plot shows the distribution of the Hek293 population after it was transfected with the following DNA parts: Hef1a-LacO:eYFP and Hef1a:mKate, both of which were constitutively active, expressing yellow and red fluorescent proteins respectively. We observe a distinct shift of approximately 83% of the population in their eYFP fluorescence (FITC channel), while we observe a 69% shift in mKate fluorescence (PE-TexasRed channel). (1B) Histogram of signal intensities of the above data. (1C) The negative control sample was transfected with plasmids containing no functional promotor-gene pairs.
We were then interested in the efficiency of multiple plasmid transfections. Previous work suggests that the probabilities of uptaking different plasmids is not independent. Cells that have been succesfully transfected with one plasmid are more likely to also have been transfected with another. To show this correlation, we decided to see what percentage of cells that took up the Hef1a:mKate plasmid also took up the Hef1a-LacO:eYFP plasmid. In order to evaluate the correlation, we took all events above the observed basal PE-TexasRed fluorescence level and examined their FITC profile. We observe in the below graph that more than 99% of the red population display yellow fluorescence above basal levels (2A). We make use of this high level of correlation later.
Figure 2: Hek293 Lipofectamine Multiplasmid Transfection Results
A

B

Figure 2: Hek293 cells were transfected with equal amounts by weight of Hef1a:mKate and Hef1a-LacO:eYFP plasmids. (2A) Scatterplot displaying fluorescence levels of events as measured by flow cytometry. Events falling below the basal threshold for red fluorescence were omitted to observe "red" population. We observe that along the FITC axis, more than 99% of the red population fall above the threshold. (2B) Histogram of the same data
Many of our circuit designs involve multiple prmoter gene pairs which means multiple plasmid transfections. We run into the issue that we cannot be sure which cells received the plasmids of interest. From this excellent correlation we can be confident that most cells with one plasmid have received others. Throughout our project, we include constitutively fluorescence expressing plasmids in transfections, allowing us to gate our data based on this constitutive color. Although this method is far from perfect, it allows us to remove untransfected background from our data and obtain a reasonable profile of our tranfected population.
Figure 3: CHO Lipofectamine Transfection Results
A

B

C

D

Figure 3: In the flow cytometry scatter plot and histogram above we see the fluorescence distribution of a population of CHO cells transfected with Lipofectamine 2000 and Hef1a_eBFP2, a constitutive blue color. We note that CHOs naturally emit autofluorescence, as seen in the small tail pointing upwards in the DAPI (blue) channel. This tail accounts for 8% of the population, so subtracting this from the 14.5% we observe in the transfected cells, we obtain approximately 6.5% transfection efficiency.
Transfection By Nucleofection
Hek293 cells display good levels of transfection efficiencies with Lipofectamine 2000. However, we found during the summer that cadherins are endogenously expressed in Hek cells, and this limits their experimental flexibility when it comes to cell-cell adhesion. CHO cells, however, do not naturally express significant levels of cadherins. Seeing also that the Notch-Delta system was previously characterized by the Elowitz group using CHO cells, we decided to port our parts into CHO cells. As documented above (3A), however, we were unable to obtain acceptable efficiency with lipofectamine in CHO cells. We then investigated alternative methods of transient transfection.Nucleofection was suggested as a good alternative. We made us of the Lonza Nucleofection technology to transfect our CHO cells. The results are shown below:
Parts Constructed
Our Favorite Parts
Type: Regulatory Length: 1275
This part encodes a promoter with low-level, constitutive activity that can be repressed by variants of the LacI transcriptional repressor. Repression by LacI-KRAB through chromatin packing is quite effective.
Type: Regulatory Length: 220
This part encodes a promoter that is inducible by variants of the Gal4 transactivator and off otherwise.
Type: Regulatory Length: 1275
This part encodes a promoter with low-level, constitutive activity that can be repressed by variants of the LacI transcriptional repressor. Repression by LacI-KRAB through chromatin packing is quite effective.
Other Parts We Like
Parts Characterizations
The characterization of newly constructed biological parts is ADD TO BLURB
List of characterizations
- Delta-Notch interaction
- rtTA3/TRE promoter
- Gal4/UAS promoter
- LacI/Hef1a-LacO repressor
- Gal4→LacI¬rtTA3→2-input AND gate
- CMV-TetO promoter
- Mnt-VP16/Mnt promoter
- CI434-VP16/CI434 promoter
Delta-Notch interaction

Explanation:
EXPLAIN HERE
rtTA3/TRE promoter


Explanation:
The rtTA3 system relies on two elements: the rtTA3 gene which encodes an activator and the TRE promoter which rtTA3 binds to. Upon doxcycline binding to rtTA3, it is able to to bind to TRE and activate expression of downstream genes. In this particular experiment, we transfected Hek293 cells with 266 ng each of Hef1a:eBFP2, Hef1a:rtTA3, and TRE:delta-mCherry constructs. Cells were then induced with varying levels of doxycycline for 48 hours before FACS. Because we generally observe >50% transfection efficiency with Lipofectamine in Hek293 cells, we took only the top 50% of cells by TxRed signal for the displayed data. We display the mean TxRed intensity for the top 50% of events verus doxycycline concentration. Control cells were given the same set of DNA but without Hef1a:rtTA3.
We can clearly observe a steep increase in red signal which levels off at dox levels above 0.5 ug/mL. Meanwhile the control cells remain at baseline, showing that doxycyline itself does not affect fluoresence levels.
Gal4/UAS promoter

Explanation:
Another activator promoter pair we make use of is the Gal4-UAS system. Gal4VP16 is an activator capable of binding to UAS promoter site and activating expression of downstream genes. Here we characterize this interaction with a set of transfections. Cells were transfected with UAS:(mKate, eYFP-FF4, eBFP2, or H2B-citrine) and Hef1a:GV16. Controls lack the Hef1a:GV16 plasmid. The above graph displays mean fluoresence in the denoted channels.
As can be clearly seen, Hef1a:GV16 results in significant activation of the UAS promoter. This results in the observed increases in mean fluoresence compared to control populations. With the exception of UAS:eBFP2, we see more than 20-fold increase in mean fluorescence.
LacI/Hef1a-LacO repressor

Explanation:
In order to implement inhibition into our circuit designs, we made use of the lacO/lacI system. lacI binds a lacO site and can inhibit transcription of downstream genes. This inhibition can be relieved by binding lacI with IPTG. In order to test this part, we constructed a Hef1a-lacO promoter which is normally constitutively active until inhibition by lacI.
As can be seen in the figure above, we see significant inhibition of eYFP expression with addition of a Hef1a:lacI construct (around 20 fold decrease). Finally, as we increase IPTG levels, we see mean yellow fluorescence increase as lacI inhibition is relieved. We also incorporate our UAS/Gal4 system by putting lacI expression under control of UAS, thus giving us a different constitutive level of lacI production. Again we observe inhibition of lacO by lacI relieved by IPTG.
Gal4-VP16→LacI¬rtTA3→2-input AND gate
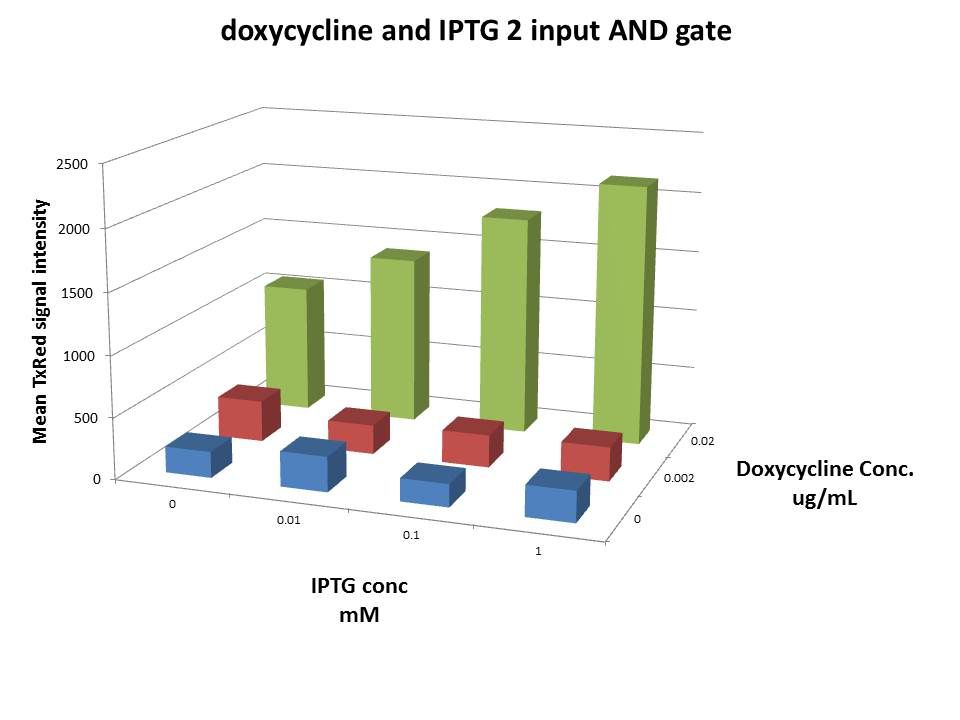

Explanation:
Here we show an experimental result of an example of what we call our internal processing module, which is essentially a 2 input chemical AND gate. Taking the individual functional transcription factor systems characterized above, we combine them into an AND gate with tewo inputs: IPTG and doxycycline levels. The system integrates the UAS/GV16, rtTA3/TRE, and lacI/lacO systems described above. IPTG is required to relieve inhibition of rtTA3 expression. rtTA3 then needs doxycycline to activate TRE. Therefore, we require both IPTG and dox to achieve expression of our final Delta-mCherry reporter.
The data show the desired 2 input AND behavior. We see maximum Delta-mCherry expression at the highest levels of both dox and IPTG. We also observe significant activation at high dox levels even in the absence of IPTG. This suggests some leakiness in the lacI inhibition, allowing for some rtTA3 to be produced and activated by dox./p>
The above result, obtained from flow cytometry by measuring the mCherry fluorescence of transfected Hek293 cells in the presence of different IPTG and doxycycline concentrations, are as we expected. In the absence of doxycycline, rtTA3 cannot activated the TRE promoter, and with increasing IPTG concentration, expressed LacI cannot inhibit the Hef1a-LacO promoter, and so we see a rise in Delta-mCherry due to this.
CMV-TetO promoter

Explanation:
The Elowitz lab has generously provided us with their stable cell lines used to characterize Notch Delta in their paper: Cis-interactions between Notch and Delta generate mutually exclusive signalling states. The cells were CHO-TRex cells stably transfected with CMV-TO:Delta-mCherry. In the absence of doxycycline, CMV-TO is inhibited by TetR. Upon doxycline addition, this inhibition is relieved. We made use of this inducible delta expression to act as "senders". In order to characterize this doxycline induction, we treated these cells with varying levels of dox and measured red fluorescence.
We display mean TxRed signal from flow cytometry versus dox levels. We clearly observe a sharp increase in signal upon dox addition which levels out.
Mnt-VP16/minCMV-4xMnt promoter

Explanation:
Mnt is similar in role to Gal4-VP16. It binds to Mnt sites and can activate expression of downstream genes. We made a minCMV-4xMnt promoter upstream of the mKate gene. Mnt presence should activate expression of mKate and thus an increase in red fluorescence. We transfected Hek293 cells with Hef1a:rtTA3, TRE:Mnt-VP16, and minCMV-4xMnt:mKate.
From the data shown, we can clearly see an increase in mean red fluorescence upon dox addition
CI434-VP16/minCMV-1xCI434 promoter

Explanation:
CI434 is another transcriptional activator that we characterized. In order to test it, we transfected Hek293 cells with Hef1a:rtTA3, TRE:CI434-VP16, and minCMV-1xCI434:mKate. This allowed us to use dox induction to activate expression of CI434 which in turn should activate mKate expression.
The results above show a clear increase in red fluorescent signal upon dox addition. We observe around a 1.25 fold increase. We believe this is an underestimate of the actual activation strength due to lack of gating and low transfection efficiency for this experiment.
Patterning Modeling Results
Semon's Modeling Results goes herePatterning Experimental Results
Here we show the results that we have obtained thus far in using our Notch-Delta system. We have tried various combinations of Hek and CHO cell co-cultures to show that the Notch-Delta system is in fact functional.Delta-Notch interaction


Explanation:
EXPLAIN HERE

Cell Adhesion Experimental Results
Cadherins are an important Calcium-dependent surface protein that can bind to same-type cadherins on other cells. The existence of different types of cadherins allows for the the development of highly organized tissues, as the sequential expression of various types of cadherins in various cells drives the development of complex three-dimensional structures.With this in mind, we brought cadherins into our project with the goal of being able to directly control cadherins in mammalian cells, leading to the formation of self-adhesive patterns when tied into the Notch-Delta and internal logic processing systems. Below we have some initial results.
Initial results.
Attributions
Our instructors were very helpful not only in giving feedback on our designs, cloning strategies, and data, but also in training us for lab work. The training for tissue culture work was conducted by Linda Stockdale in the Griffiths lab. Gibson assembly techniques and FACS training from Deepak Mishra, one of our instructors.
Other than the initial training, all work was done by our undergraduate team.
Special credit belongs to Semon Rezchikov for simulations and modeling, Jenny Cheng for animations, and Tiffany Huang for wiki design.
 "
"