RecA Project
From 2011.igem.org
(→RecA Co-Protease) |
|||
| Line 30: | Line 30: | ||
<p>In order for RecA to still detect damaged DNA, but not repair it, RecA needed to cleave the C1 repressor in order to prevent repression of the PR promoter in our device. If RecA was allowed to recombine the DNA it would destabilize our plasmid and prevent our device from working.</p> | <p>In order for RecA to still detect damaged DNA, but not repair it, RecA needed to cleave the C1 repressor in order to prevent repression of the PR promoter in our device. If RecA was allowed to recombine the DNA it would destabilize our plasmid and prevent our device from working.</p> | ||
| - | |||
==Summary of RecA Mutations== | ==Summary of RecA Mutations== | ||
| Line 45: | Line 44: | ||
**TTC ⇒ TTT (Codon usage bias increases: .49 to .51) | **TTC ⇒ TTT (Codon usage bias increases: .49 to .51) | ||
| - | + | ==Test Circuit== | |
| - | + | ||
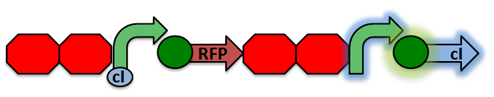
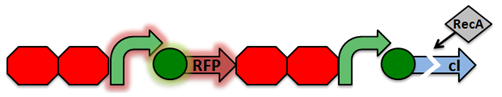
'''RecA Test Circuit:''' The cI repressor is constitutively produced and binds to the repressible promoter under normal settings.[[File:RecA Test Circuit1.png|center|]] | '''RecA Test Circuit:''' The cI repressor is constitutively produced and binds to the repressible promoter under normal settings.[[File:RecA Test Circuit1.png|center|]] | ||
| - | Activated RecA then cleaves the | + | <p>To test our RecA mutant we developed a test circuit which included a double terminator followed by a driver (C1 promoter, RFP, double terminator), constitutive promoter, and a C1 repressor. The C1 repressor is constitutive produced and binds to the repressible promoter under normal settings. Activated RecA then cleaves the C1 repressor, causing transcription of RFP. However, if RecA exhibits recombinant activity, then the homologous double terminator regions will recombine. This would cause the deletion of of the repressible promoter and RFP and allow us to identify if our mutation was successful or unsuccessful.</p> |
[[File:RecATest2.png|center|]] | [[File:RecATest2.png|center|]] | ||
| Line 57: | Line 55: | ||
<p>When using the double primer method of site directed mutagenesis, only one base pair can be mutated at one time. Thus, the Penn State team redefined their goal regarding RecA1 mutagenesis in order to expand their data set. By mutating each site at one time, the Penn State team can use the RecA test circuit to determine the effectiveness of each mutation as well as each combination of mutations. The EcoRI and PstI sites had to be mutated in every RecA plasmid in order for BioBrick assembly, which was needed to put the RecA test circuit together, to work correctly. Once these sites were mutated, the plasmid containing these two mutations was then used to mutate the Lys, Arg, and RecA activation sites individually. Once these sites were mutated, the plasmids were used to mutate a different site that did not already exist in the plasmid. Finally, the final site was mutated, resulting in a plasmid that contained all five mutations in RecA1 to form RecA. This left seven total mutated RecA1 plasmids that could be tested using the RecA test circuit. The Penn State mutagenesis plan can be summarized by the below picture.</p> | <p>When using the double primer method of site directed mutagenesis, only one base pair can be mutated at one time. Thus, the Penn State team redefined their goal regarding RecA1 mutagenesis in order to expand their data set. By mutating each site at one time, the Penn State team can use the RecA test circuit to determine the effectiveness of each mutation as well as each combination of mutations. The EcoRI and PstI sites had to be mutated in every RecA plasmid in order for BioBrick assembly, which was needed to put the RecA test circuit together, to work correctly. Once these sites were mutated, the plasmid containing these two mutations was then used to mutate the Lys, Arg, and RecA activation sites individually. Once these sites were mutated, the plasmids were used to mutate a different site that did not already exist in the plasmid. Finally, the final site was mutated, resulting in a plasmid that contained all five mutations in RecA1 to form RecA. This left seven total mutated RecA1 plasmids that could be tested using the RecA test circuit. The Penn State mutagenesis plan can be summarized by the below picture.</p> | ||
| + | |||
| + | ==Results== | ||
| + | As of the wiki freeze, the Penn State team has been able to make the following mutations: | ||
| + | <br>EcoRI+PstI+RecA activation</br> | ||
| + | <br>EcoRI+PstI+RecA activation+Lys</br> | ||
Revision as of 06:08, 27 September 2011

RecA Project Sensor Project Reporter Project
Contents |
RecA Co-Protease
RecA is a protein used by E. coli to repair and maintain DNA. Our project aims to use RecA to detect damaged DNA and then prevent it from repairing DNA. Instead of repairing the DNA we aim to connect it to a Reporter to identify damaged DNA.
The normal laboratory strain of E. coli RecA, DH10B contains an inactivated form of RecA known as RecA1. RecA 1 is deficient in all known function of the RecA gene specifically in ATPase activity, binding with DNA in the presence of ATP, and changing conformation in the presence of ATP and repressor cleavage. In order to restore RecA1 to RecA a series of mutations must occur. The mutation occurs at amino acid 160, the adenine needs to be mutated to guanine to change RecA1 to RecA and restore its functions.
To do this we first extracted the RecA1 DNA from E coli obtaining our genomic DNA. We then had to insert the genomic DNA onto a plasmid. We first tried to use a chloramphenicolresistant plasmid for the RecA1 clone as our plasmid, however the genomic DNA did not take the chloramphenicolresistant plasmid, so instead we tried a kanamycin resistant plasmid. After several cloning attempts, the Penn State team finally had RecA1 inside a plasmid.
In order to use the standard bio-bricking techniques we also had to remove naturally occurring enzymatic restriction sites. The Pst1 site CTGCAG needed to be converted to CTGCAA. Then the EcoR1 site GAATTC needed to be converted to GAATTT.
We also focused on how to mutate RecA so that it would detect damaged DNA without repairing the DNA. After literature research we identified two amino acids in the RecA sequence that were known to affect RecA’s ability to use recombinase to repair the DNA. These sites were Arginine 243 and Lysine 286. Research suggested that changing Arginine 243 to Glutamine, and Lysine 286 to Asparagine would remove recombinase activity from RecA.
In order for RecA to still detect damaged DNA, but not repair it, RecA needed to cleave the C1 repressor in order to prevent repression of the PR promoter in our device. If RecA was allowed to recombine the DNA it would destabilize our plasmid and prevent our device from working.
Summary of RecA Mutations
Activation of RecA
- Point Mutation at b.p 720: A ⇒ G
Removal of Recombinase Activity
- Arginine 243 ⇒ Glutamine
- Lysine 286 ⇒ Asparagine
In order to use the standard bio-bricking techniques, we also had to remove these naturally occurring enzymatic restriction sites
- PST1 Site [CTGCAG]
- CAG ⇒ CTG (Codon usage bias decreases: .69 to .31)
- EcoR1 Site [GAATTC]
- TTC ⇒ TTT (Codon usage bias increases: .49 to .51)
Test Circuit
RecA Test Circuit: The cI repressor is constitutively produced and binds to the repressible promoter under normal settings.To test our RecA mutant we developed a test circuit which included a double terminator followed by a driver (C1 promoter, RFP, double terminator), constitutive promoter, and a C1 repressor. The C1 repressor is constitutive produced and binds to the repressible promoter under normal settings. Activated RecA then cleaves the C1 repressor, causing transcription of RFP. However, if RecA exhibits recombinant activity, then the homologous double terminator regions will recombine. This would cause the deletion of of the repressible promoter and RFP and allow us to identify if our mutation was successful or unsuccessful.
Method
The RecA group focused its resources on mutating RecA1 as described above. To perform these mutations, site directed mutagenesis was performed on the RecA1 plasmid. The single primer method was used in an attempt to perform all five mutations at one time. The protocol for this procedure can be found on the protocols page, and was taken from the Knight Lab openwetware page. After a summer of failure with this method (only one mutation in three months), the Penn State team switched to the double primer method using a protocol based off of the protocol from the Richard Lab openwetware page. The modified protocol can be found on the protocols page.
When using the double primer method of site directed mutagenesis, only one base pair can be mutated at one time. Thus, the Penn State team redefined their goal regarding RecA1 mutagenesis in order to expand their data set. By mutating each site at one time, the Penn State team can use the RecA test circuit to determine the effectiveness of each mutation as well as each combination of mutations. The EcoRI and PstI sites had to be mutated in every RecA plasmid in order for BioBrick assembly, which was needed to put the RecA test circuit together, to work correctly. Once these sites were mutated, the plasmid containing these two mutations was then used to mutate the Lys, Arg, and RecA activation sites individually. Once these sites were mutated, the plasmids were used to mutate a different site that did not already exist in the plasmid. Finally, the final site was mutated, resulting in a plasmid that contained all five mutations in RecA1 to form RecA. This left seven total mutated RecA1 plasmids that could be tested using the RecA test circuit. The Penn State mutagenesis plan can be summarized by the below picture.
Results
As of the wiki freeze, the Penn State team has been able to make the following mutations:
EcoRI+PstI+RecA activation</br>
EcoRI+PstI+RecA activation+Lys</br>
 "
"