results
Precipitator
Green light receptor
We could successfully amplify the gene sequences for CcaS and CcaR and the promotor region PcpcG via
PCR from the whole Synechocystis sp. PCC6803 genome.
Later two parts ([http://partsregistry.org/Part:BBa_K608101 BBa_K608101] , [http://partsregistry.org/Part:BBa_K608102 BBa_K608102]), were inserted into the iGEM-Vector pSB1C3 and send to the registry.
Twice we tried to clone the promotor region PcpcG into a vector, but had no insert at all.
After a summer full of cloning we ran out of time to assemble
the green light receptor cassette like we planned to.
red light receptor
In August we decided to drop the red light receptor system.
The main reason beeing Jakob having to leave the team to start studying in Strasbourg.
So the FreiGEM team shrunk to five people.
Still we did submit one part for this system which is [http://partsregistry.org/Part:BBa_K608151 BBa_K608151]
the translational unit of pcyA
We talked to some of the iGEM teams like TU Munich also working with this system
and we are sure that the red light receptor is not lost.
blue light receptor
The handling with the LOV domain and TrpR domain was very difficult.
On the one hand it was difficlut to digest the parts, because there was a SpeI restriction site in the tryptophan repressible promoter region. So we first had to mutate SpeI into NehI restriction site to digest our parts without digesting the promotor. In the beginning we also tried the Gibson assembly. Primer design was quite difficult because of the lenght, the usually very high annealing temperature and unspecifity of the sequence combined to the mutations we had to include. These hassel leaded to trouble to amplify the Lov domain.The 3A-assembly with the mutated parts wasn´t successful either. We only cloned the Not-Gate in the pSB1C3 vector. We tried this experiment with different parameters but we weren´t successful.
Lysis cassette
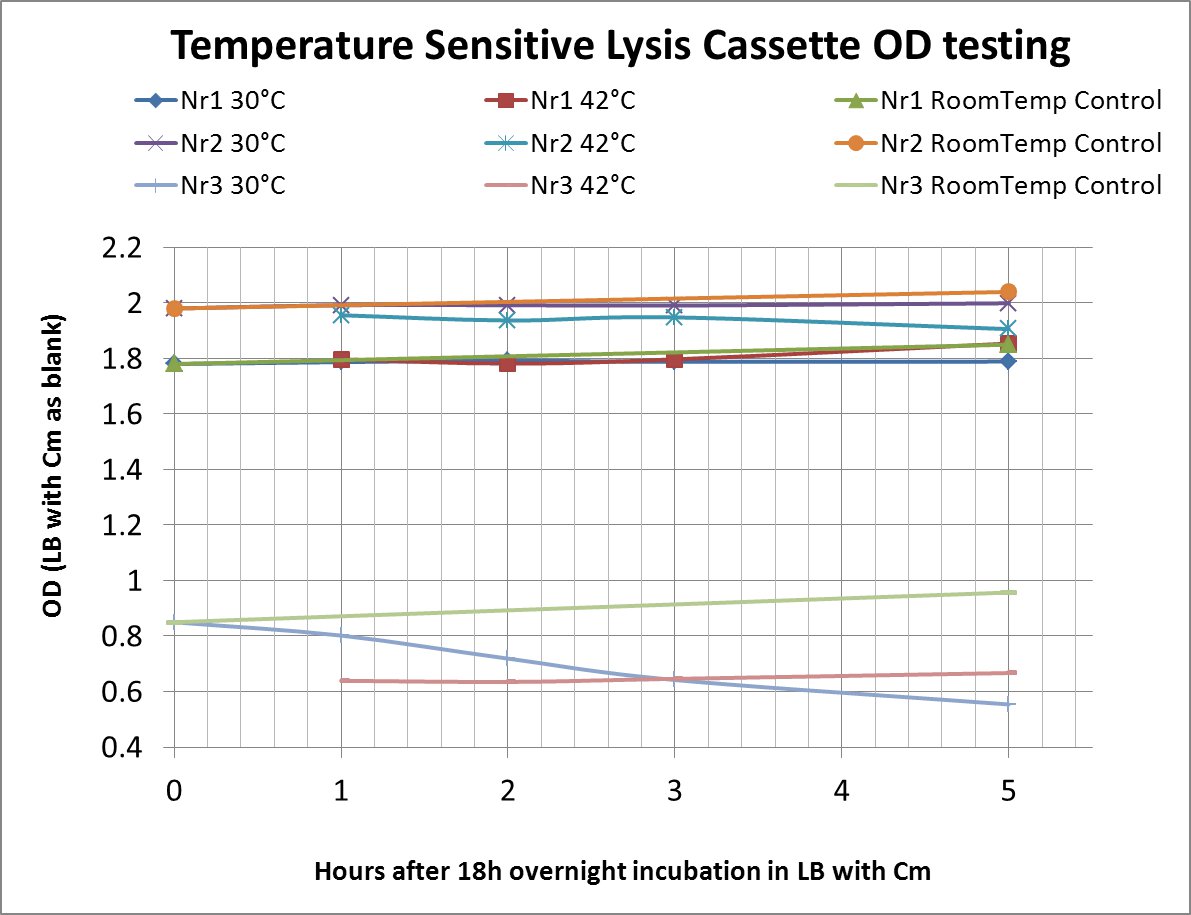
After a lot of mishappenings concerning the 2 parts making up the temperature sensitive lysis cassette, we made a last attempt to ligate them (link to workbook) and transform the novel composite over the last weekend before the Wiki-freeze. After putative positive colonies were picked, we managed to perform a last-minute, "blind" -ie not knowing if the sequence of the insert was correct-, and preliminary OD-measurement testing.
We later verified the part sequence. Since we cannot upload .ab1 files, we made 2 base-call files from them without changing absolutely anything (that means that there are some false bases at the beginning and end of every file).
- Forward Primer
- Reverse Primer
The OD values of the measurement are shown in the chart (please do bear in mind that this was a measurement done under deadline-stress, without the possibility to plan and perform something more concrete before the Wiki-Freeze)
- LB with Cm (Chloramphenicol) was used as a blank before every (hourly) measurement
Nr1: Insert is only part ([http://partsregistry.org/Part:BBa_K608351 BBa_K608351])
Nr2: Insert is only part ([http://partsregistry.org/Part:BBa_K608352 BBa_K608352])
Nr3: Both parts were correctly assembled (1:([http://partsregistry.org/Part:BBa_K608351 BBa_K608351]) + 2:([http://partsregistry.org/Part:BBa_K608352 BBa_K608352]))
Therefore the temperature sensitive lysis cassette seems to be pretty temperature-sensitive but also functioning properly. More tests are planned if time allows (ie wiki-unfreeze ;) )
Parts submitted to the registry
All our results are documented in our notebook and summarized in the partsregistry.
Here we shortly describe what happened to give you an idea about the current status of the different subprojects.
Please follow the links for more information.
Precipitator
[http://partsregistry.org/Part:BBa_K608404 BBa_K608404]
IPTG-inducible Promoter with plastic binding domain-tagged GFP
[http://partsregistry.org/Part:BBa_K608406 BBa_K608406]
Precipitator
The Precipitator is a new artificially designed LRR protein. It is meant as protein that binds Nickel ions with Histidines grouped on its surface. The bound Nickel can then precipitate His-tagged proteins. In our Lab in a Cell it should function as an adaptor between the plastic surface of pipettes and the His-tagged protein. Please look at our detailed description of the design layout in our modeling section. The sequence was synthesised and cloned into the iGEm vector. The submitted sequence was fully confirmed by sequencing.
[http://partsregistry.org/Part:BBa_K608407 BBa_K608407]
Precipitator fused with GST tag
The Precipitator was fused with the C-terminus of our GST tag in order to extract and further test the construct for its Nickel binding affinity. We successfully cloned it together using the Gibson Assembly and then subsequently pasted it into the iGEM vector. The submitted sequence was partially confirmed by sequencing.
[http://partsregistry.org/Part:BBa_K608408 BBa_K608408]
GST-tag
The GST-tag was PCR amplified from a pGEX vector with overhang primers including the iGEM restriction sites and then pasted into the iGEM vector. To verify the functionality of the construct we cloned it before a GFP sequence and expressed it with an IPTG inducible vector. Results see partsregistry page. The submitted sequence was partially confirmed by sequencing.
Green light receptor
[http://partsregistry.org/Part:BBa_K608101 BBa_K608101]
CcaR, green light response regulator
The green light response regulator CcaR was amplified via PCR from
the whole Synechocystis sp. PCC 6803 genome and inserted into the pSB1C3 vector.
[http://partsregistry.org/Part:BBa_K608102 BBa_K608102]
CcaS, green light receptor
[http://partsregistry.org/Part:BBa_K608151 BBa_K608151]
translational unit of pcyA
Lysis cassette
[http://partsregistry.org/Part:BBa_K608351 BBa_K608351]
(correct) temperature sensitive promoter
[http://partsregistry.org/Part:BBa_K608352 BBa_K608352]
Bacteriophage Lysis Cassette with RBS
commons
At the beginning we had to assemble promoters and ribosome binding sites of different "strengths" (ie consensus identities) in order to ensure proper and controlled expression of our parts.
The cloning procedure was time-consuming (circa two weeks), so we decided to
send our constructs to the registry. Herewith we hope to help others save some time.
We called these constructs PR to abbreviate "Promoter and RBS"
[http://partsregistry.org/Part:BBa_K608002 BBa_K608002]
strong Promoter and strong RBS
[http://partsregistry.org/Part:BBa_K608003 BBa_K608003]
strong Promoter , medium RBS
[http://partsregistry.org/Part:BBa_K608004 BBa_K608004]
strong Promoter , weak RBS
[http://partsregistry.org/Part:BBa_K608005 BBa_K608005]
medium Promoter , strong RBS
[http://partsregistry.org/Part:BBa_K608006 BBa_K608006]
medium Promoter , medium RBS
[http://partsregistry.org/Part:BBa_K608007 BBa_K608007]
medium Promoter , weak RBS
To quantify the strength of our PR-constructs we cloned GFP and RFP behind it
and quantified the protein output.
[http://partsregistry.org/Part:BBa_K608008 BBa_K608008]
constitutive strong promoter with medium RBS and GFP
[http://partsregistry.org/Part:BBa_K608009 BBa_K608009]
Strong promoter with weak RBS and GFP
[http://partsregistry.org/Part:BBa_K608010 BBa_K608010]
Medium promoter with strong RBS and GFP
[http://partsregistry.org/Part:BBa_K608011 BBa_K608011]
Medium promoter with medium RBS and GFP
[http://partsregistry.org/Part:BBa_K608012 BBa_K608012]
Medium promoter with weak RBS and GFP
[http://partsregistry.org/Part:BBa_K608013 BBa_K608013]
strong Promoter with strong RBS and RFP
[http://partsregistry.org/Part:BBa_K608014 BBa_K608014 ]
PR with RFP
[http://partsregistry.org/Part:BBa_K608015 BBa_K608015]
PR with RFP
[http://partsregistry.org/Part:BBa_K608016 BBa_K608016]
PR with RFP
[http://partsregistry.org/Part:BBa_K608017 BBa_K608017]
PR with RFP
[http://partsregistry.org/Part:BBa_K608018 BBa_K608018]
PR with RFP
 "
"
 Contact
Contact