Team:Potsdam Bioware
From 2011.igem.org
| Line 1: | Line 1: | ||
| - | + | {{:Team:Potsdam_Bioware/Head}}{{:Team:Potsdam_Bioware/jquery}}{{:Team:Potsdam_Bioware/menu_home}} | |
==<center>Modification, Selection and Production of Cyclic Peptides for Therapy</center>== | ==<center>Modification, Selection and Production of Cyclic Peptides for Therapy</center>== | ||
Revision as of 10:00, 10 September 2011
Contents |
Modification, Selection and Production of Cyclic Peptides for Therapy
Abstract
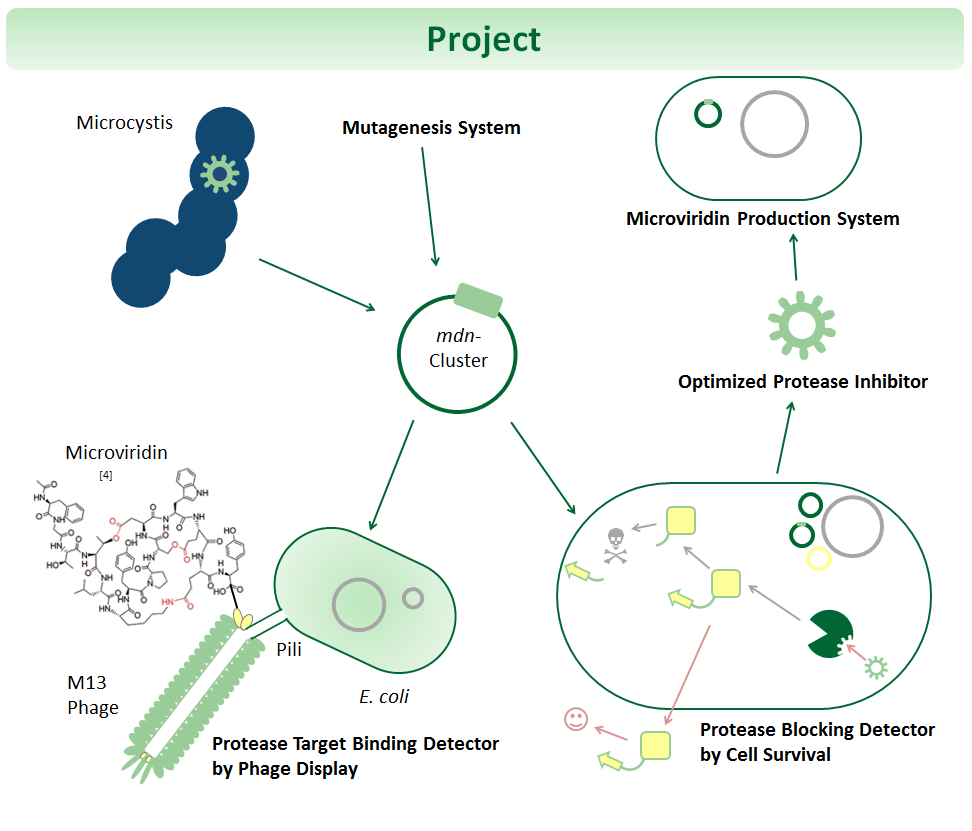
One key task of biopharmaceuticals is the binding and blocking of deregulated proteins. Towards this goal, we mutate and select microviridins, which are tricyclic depsipeptides from cyanobacteria. They are small but stable due to their post-translational side-chain crosslinking. Microviridins have a high potential for therapy as they can block disease-relevant proteases. Yet, the possibilities of cyclic peptides are largely untapped since genetic systems for optimization are not well established. Thus, we developed synthetic systems for the mutation, selection and production of such peptides. We use the 6.5 kb microviridin (mdn) gene cluster cloned in E. coli plasmids, established random mutagenesis and generated focused libraries of microviridins. For selection against a panel of proteases, we are applying and testing phage display, and we are constructing a novel in-vivo selection device, which links protease blocking to antibiotic resistance. Our systems, including the 6.5 kb cluster, adhere to the BioBrick standards.
Safety and Security Assessment
Our iGEM project requires only the handling of the non-pathogenic, non-adherent Escherichia coli K12 and B strains and the well-established filamentous phage. Both, the bacteria and the phage are commonly used chassis in laboratories and pose no risk when handled according to the mandatory rules. As all of us are well briefed about laboratory safety and biohazard regulations we follow these at all times. In Germany, work with genetically modified organisms is regulated by the ‘Law on Gene Technology’ (Gesetz zur Regelung der Gentechnik, GenTG). According to these rules, the responsible governmental authorities of the state of Brandenburg have been notified about our work. Following these rules, there should not be a significant danger neither to the environment nor to team members.
The most important issues we discussed are the consequences of the error-prone PCR we use to modify our parts. We tried to estimate the chances of generating highly toxic proteins. Surveying the literature, we found several reports about natural variants of microviridins and one rational mutational study, but no reports on toxic effects. As cyanobacteria can also produce toxic compounds (non-ribosomal peptides named microcystins) toxicity testing is well established in the cyanobacteria research community, and obviously, testing did not identify toxic effects. Therefore, we assume that our mutations will not have any hazardous effects. Additionally, the obtained, constructed, and planned plasmids contain only previously described parts without any known risk potential. Therefore, as far as we can foresee, our constructed BioBricks will not have or trigger any toxic effects or be critical in any way for the environment. This means that only a negligible risk arises from our used methods and constructs to the environment, the public and the team members. Last but not least, we do not see any particular danger of abuse or other security threat of our work, since it is specifically addresses scientific questions. It is our goal that the health of mankind and the environment benefit from our research.
Safety Questions
- 1. Would any of your project ideas raise safety issues in terms of: researcher safety, public safety, or environmental safety?
- No, our project is not raising any of these safety issues.
- 2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes, did you document these issues in the Registry? how did you manage to handle the safety issue? How could other teams learn from your experience?
- No, our BioBrick parts or devices are not going to raise any safety issues.
- 3. Is there a local biosafety group, committee, or review board at your institution? If yes, what does your local biosafety group think about your project? If no, which specific biosafety rules or guidelines do you have to consider in your country?
- In Germany, work with genetically modified organisms is regulated by the ‘Law on Gene Technology’ (Gesetz zur Regelung der Gentechnik, GenTG). According to these rules, the responsible governmental authorities of the state of Brandenburg have been notified about our work. Our work was classified as biosafety level 1.
- 4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
- So far biosafety assessment is primarily based on the characterization of wild-type devices or systems. For Synthetic Biology these rules should be extended to better represent the variations made by synthetic biology approaches. In addition to the continuous evaluation of safety and security, a section on technological impact assessment should be added.
 "
"