green light receptor
Qickchange PCR of CcaS
Investigators:Julia
repeating experiment from___, because we had no positive transformation, probably chosen wrong annealing temperature before.
blue light receptor
PCR
Investigators: Sophie
As our last PCRs of the LovTAP with the Gibson overhangs didn't work well, we now try a PCR with primers without overhangs for Gibson assembly.
| Name: Sophie
| Date: 01.08.11
|
| Continue from Experiment: PCR (Date): 27.07.11
(Name): Sandra, Sophie
|
| Project Name: Blue Light
|
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl
| H20
| Name
|
| 10µl
| 5x Phusion Buffer
| of Primer
|
| 2.5µl
| Primer fw
| P35
|
| 2.5µl
| Primer dw
| P36
|
| 1µl
| dNTPs
| of Template DNA
|
| 1µl
| DNA-Template
| M35 (LovTAP)
|
| 0.5 µl
| Phusion (add in the end)
|
|
What program do you use?
LovTAP ohne Ueberhaenge
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
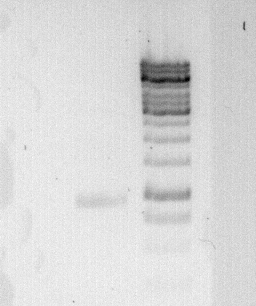
How did you label the PCR-Product, where is it stored and what do you do next?
Labelled: M35 short; stored in Gibson Stuff box.
red light receptor
3A-assembly with pcyA and the terminator BBa_1006
Investigators:Julia
1.Digestion:
terminator (S21a), 26µl of the miniprep+ 12,1 µl H2O, cut with XbaI&PstI
pcyA (part BBa_I15009), 3 µl of miniprep + 35µl H2O, cut with EcoRI&SpeI
Vector-backbone (10 µl) pSB1T3, cut with EcoRI,PstI & DpnI
to each reation 5µl BSA(10x) and 5µl NEB buffer4 were added, digested at 37°C for two hours, inactivated at 80°C for 20min.
2.Ligation
2µl of digested pcyA,terminator and vector were added to 11µl H2O,2µl ligase buffer and 1µl T4 ligase.
Incubated at room temperature for 35 min, inactivated at 80°C for 20 min.
3.Transformation
Lysis cassette
Phage Lysis Cassette (K124017) + RBS (B0034)
Investigators: Theo
the absence of a RBS (ribosome binding site) between the lamba cI regulated Promotor (R0051 of K098995) and the holin ATG of K124017
Precipitator
PCR
| Name: Sophie
| Date: 01.08.11
|
| Continue from Experiment: replay of PCR 19.07.11 (Date) 19.07.11
(Name) Ruediger
|
| Project Name: GFP Pbd
|
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl
| H20
| Name
|
| 10µl
| 5x Phusion Buffer
| of Primer
|
| 2.5µl
| Primer fw
| P28
|
| 2.5µl
| Primer dw
| P18 / P19/ P20
|
| 1µl
| dNTPs
| of Template DNA
|
| 1µl
| DNA-Template
| S 14 (GFP)
|
| 0.5 µl
| Phusion (add in the end)
|
|
What program do you use?
Ruediger PCR (modified by Tobi and me. First 10 cycles with 55°C, next cycles with 62°C annealing temperature.
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
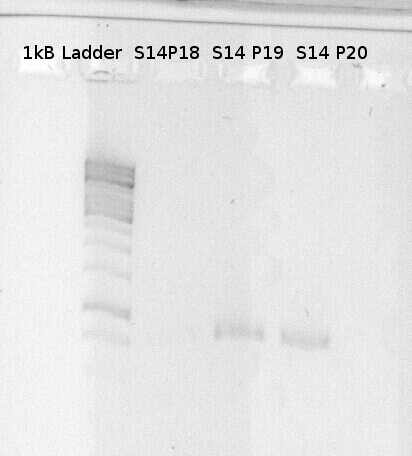
How did you label the PCR-Product, where is it stored and what do you do next?
 "
"
 Contact
Contact 




