Team:Harvard/Template:NotebookDataJuly
From 2011.igem.org
(Difference between revisions)
| Line 9: | Line 9: | ||
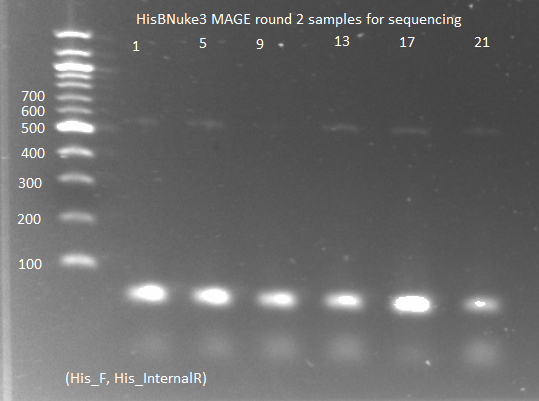
**sent to Genewiz to be sequenced with His3_F primer | **sent to Genewiz to be sequenced with His3_F primer | ||
| - | [[File: | + | [[File: HARV2011.07.01.hisBnuke3MAGE2_for_seq2(labeled).png|thumb|none|HisBNuke3 MAGE round 2 samples for sequencing 7/1/11]] |
'''Lambda Red recombination results:''' | '''Lambda Red recombination results:''' | ||
| Line 21: | Line 21: | ||
**157.4ng/µL 260/280=1.9; 129.2ng/µL 260/280=1.88 | **157.4ng/µL 260/280=1.9; 129.2ng/µL 260/280=1.88 | ||
| - | [[File: | + | [[File: HARV2011.07.01.pZE21Gbackbonesuccess(labeled).png|thumb|none|pZE21G backbone 7/1/11]] |
'''Selection system plates:''' | '''Selection system plates:''' | ||
| Line 56: | Line 56: | ||
**Because of KAPA shortage, only ran samples 6-8 from 100µL 6/29 plate and 6-7 from 2mL 6/29 plate plus (ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture as a control (should produce a band around 300bp, while the insert would make the band 2-3kb) | **Because of KAPA shortage, only ran samples 6-8 from 100µL 6/29 plate and 6-7 from 2mL 6/29 plate plus (ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture as a control (should produce a band around 300bp, while the insert would make the band 2-3kb) | ||
**Ran on E-Gel with team TolC's samples, but there was an issue loading the gel, so also ran separately on another gel. Unfortunately most of the PCR reactions strangely did not work, but two of the samples appeared not to have the insert (see below). | **Ran on E-Gel with team TolC's samples, but there was an issue loading the gel, so also ran separately on another gel. Unfortunately most of the PCR reactions strangely did not work, but two of the samples appeared not to have the insert (see below). | ||
| - | [[File: | + | [[File:HARV2011.07.05.kanZFBwp_tolC+kanZFBinsert_check.png|200px|thumb|none|2011.07.05.kanZFBwp_tolC+kanZFBinsert_check.ai]] |
| Line 111: | Line 111: | ||
**(ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture used as a control | **(ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture used as a control | ||
| - | [[File: | + | [[File: HARV2011.07.06.kanZFBinsertcheck1(labeled).png|thumb|none|1529620 locus PCR 1 7/6/11]] |
| - | [[File: | + | [[File: HARV2011.07.06.kanZFBinsertcheck2(labeled).png|thumb|none|1529620 locus PCR 2 7/6/11]] |
*NM media has 1% of the glucose concentration written in the protocol, it is 0.4mg/ml instead of 40mg/ml. Nevertheless, our overnight culture of pKD46 grew successfully so we will continue to use the media unless other problems arise. | *NM media has 1% of the glucose concentration written in the protocol, it is 0.4mg/ml instead of 40mg/ml. Nevertheless, our overnight culture of pKD46 grew successfully so we will continue to use the media unless other problems arise. | ||
| Line 121: | Line 121: | ||
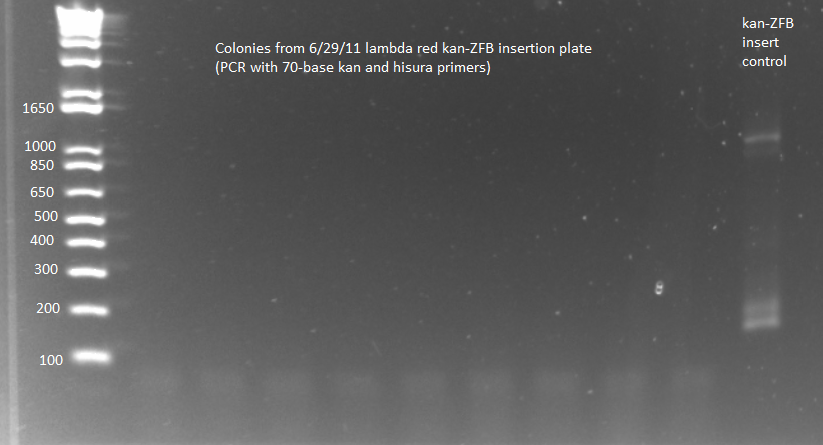
**results from 2 E Gels: PCR not very successful. Colonies showed no bands while the control showed only a side product too small to be the full insert. | **results from 2 E Gels: PCR not very successful. Colonies showed no bands while the control showed only a side product too small to be the full insert. | ||
| - | [[File: | + | [[File: HARV2011.07.06.kanZFBinsertcheck_longprimer1(labeled).png|thumb|none|Kan-ZFB check using kan and hisura primers 7/6/11]] |
*Grew up 24 more kan-ZFB lambda red recombined colonies (12 from 100µL 6/29 plate, 12 from 2mL 6/29 plate) in LB+kan in 96 well plate for PCR tomorrow | *Grew up 24 more kan-ZFB lambda red recombined colonies (12 from 100µL 6/29 plate, 12 from 2mL 6/29 plate) in LB+kan in 96 well plate for PCR tomorrow | ||
| Line 146: | Line 146: | ||
**4°C forever | **4°C forever | ||
| - | [[File: | + | [[File:HARVKAN-ZFB-WP2 Initial Attempt.png|200px|thumb|none|KAN-ZFB-WP]] |
===Web Design=== | ===Web Design=== | ||
*Project description | *Project description | ||
*Web design brainstorming;exploring past designs and templates</div> | *Web design brainstorming;exploring past designs and templates</div> | ||
 "
"