Team:Caltech/Week 5
From 2011.igem.org
| Line 74: | Line 74: | ||
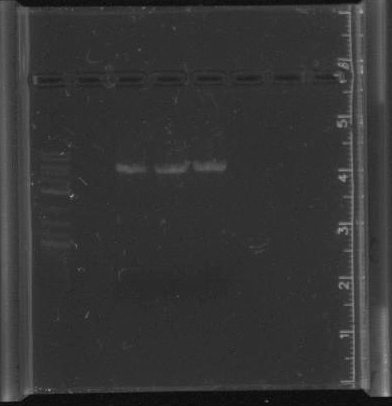
Try to PCR the miniprep DNA with the pSB3C5 primers<br/> | Try to PCR the miniprep DNA with the pSB3C5 primers<br/> | ||
Do Gibson with the PCR parts that worked</p> | Do Gibson with the PCR parts that worked</p> | ||
| + | |||
| + | '''Labels for 16s sequencing PCR:''' | ||
| + | * 9-1 (1/10): 9a | ||
| + | * 9-1 (1/100): 9b | ||
| + | * 9-1 (1/1000): 9c | ||
| + | * 10-3 (1/10): 10a | ||
| + | * 10-3 (1/100): 10b | ||
| + | * 10-3 (1/1000): 10c | ||
| + | * Control 1: C1 | ||
| + | * Control 2: C2 | ||
| + | |||
| + | '''Labels for PCR Miniprep:''' | ||
| + | * pSB3C5 (1/100)- HA | ||
| + | * pSB3C5 (1/100)- HB | ||
| + | * pSB3C5 (1/100)- HC | ||
| + | * pSB3C5 (1/1000)- TA | ||
| + | * pSB3C5 (1/1000)- TB | ||
| + | * pSB3C5 (1/1000)- TC | ||
===Results=== | ===Results=== | ||
Revision as of 20:05, 15 July 2011
|
Project |
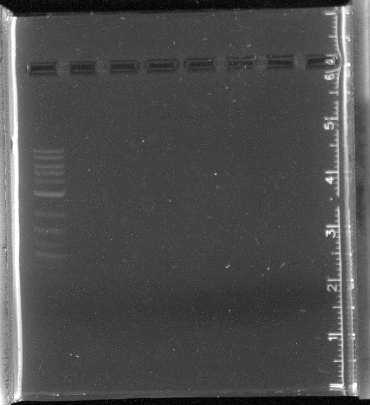
July 11Miniprep pSB4A5 ResultsThe PCR of parts for pNT003 was successful except for the terminator (B0014), as there is no band in that lane. July 12Transformed competent cells with pSB3C5. Ran PCR of parts for pNT001 and pNT002 Gibson assembly. Started pulsed field gel electrophoresis of some of our LA River DNA obtained by the Mo Bio kits in week 2. ResultsAll of the parts for pNT001, pNT002 and B0014 were PCR'd with primers for Gibson assembly. All lanes except for lanes 4 and 5 had single bands, indicating that the parts were successfully amplified. July 13Retrieved and imaged pulsed field gel. ResultsThe pulsed field gel had to be restained after running. The ladder and control appeared, but none of our LA River sample DNA. Since the ladder ranges from 5kb to 50 kb, we should be able to see some smears in the experimental lanes, as we ran a short gel last week and found that the sample DNA was greater than 10kb. Since the ladder does not seem well separated, we will try running the gel again with different parameters. The pSB3C5 had many colonies, but none of them are red. This is strange because the plasmid contains BBa_J04450 as an insert, a RFP. We are replating on a new chlor plate and starting overnights to send off for sequencing to make sure the plasmid is okay. PCR Purifications for Gibson
July 14Miniprep overnights and send off for sequencing Labels for 16s sequencing PCR:
Labels for PCR Miniprep:
ResultsThe minipreps of the overnight cultures all had very low concentrations of DNA.
July 15ResultsGibson Plates
Perhaps our cells are not being very competent again. We should do a 20 ul reaction instead of a 10.5 ul reaction so we can retransform if necessary. Also, we should change amounts of DNA in the reaction. We've been diluting the DNA and trying to get all the parts within a 10 ng range.
|
 "
"