Team:Harvard/Results
From 2011.igem.org
| Line 24: | Line 24: | ||
=Genome-Based One-Hybrid Selection Strain= | =Genome-Based One-Hybrid Selection Strain= | ||
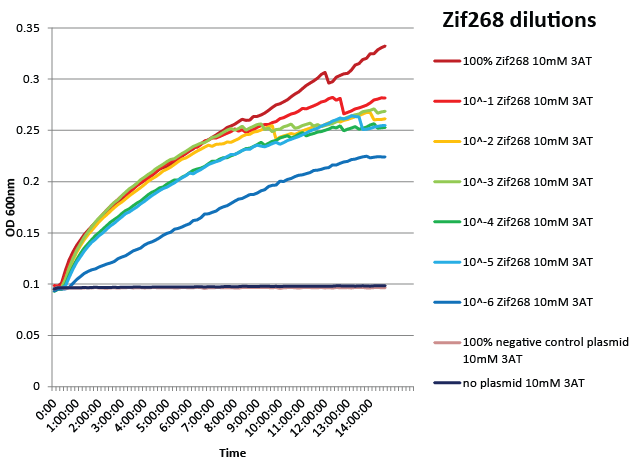
| - | We designed a one-hybrid metabolic system that was entirely genome-based. Using multiplex automated genome engineering (MAGE) and lambda red, we knocked out HisB, PyrF, and rpoZ; inserted a kanamycin cassette-zinc finger binding site-His3-URA3 construct into the 1529620 locus; and changed the zinc finger binding site directly on the genome. The strain was fully characterized and was sensitive enough to recognize a valid zinc finger when diluted as much as one into one million of negative controls. See [https://2011.igem.org/Team:Harvard/Project/Test# | + | We designed a one-hybrid metabolic system that was entirely genome-based. Using multiplex automated genome engineering (MAGE) and lambda red, we knocked out HisB, PyrF, and rpoZ; inserted a kanamycin cassette-zinc finger binding site-His3-URA3 construct into the 1529620 locus; and changed the zinc finger binding site directly on the genome. The strain was fully characterized and was sensitive enough to recognize a valid zinc finger when diluted as much as one into one million of negative controls. See [https://2011.igem.org/Team:Harvard/Project/Test#Fine-tuning_the_one-hybrid_selection_system here] for more details. |
Revision as of 05:25, 25 October 2011
Overview | Biobricks | Source Code | Acknowledgments | Accomplishments
Contents |
Novel Zinc Fingers
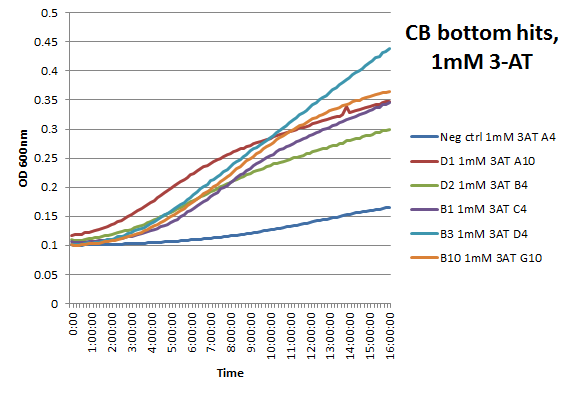
We did not have time to test all 6 targets as planned: we chose to focus on zinc finger binders to DNA tripplet TGG, which is found in the color blindness gene bottom target.
We assembled the Color Blindness Bottom library, transformed it into the proper selection strain, and plated on incomplete media with varying concentrations of 3-AT. We saw colonies form, with more on the less stringent plates (NM) and fewer colonies on the higher 3-AT concentrations. Several colonies were picked and tested under varying concentrations of 3-AT in plate reader overnight, and there was a clear difference in growth between the colonies and the negative control (CB bottom binding site with Zif268).
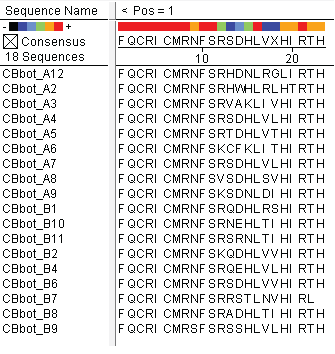
These colonies were then sequenced to discover which of our library zinc fingers was successfully binding to the 5'-GTG GGA TGG-3' sequence. So far, preliminary results indicate that 15 of the colonies have sequences consistent with the DNA sequences on the chip that was submitted, meaning that we have discovered up to 15 novel zinc fingers. The sequences consistent with the chip are CBBot_A1, A3, A4 (same as A7), A6, A8, A9, B1, B10, B11, B2, B4, B6, B7, B8, and B9.
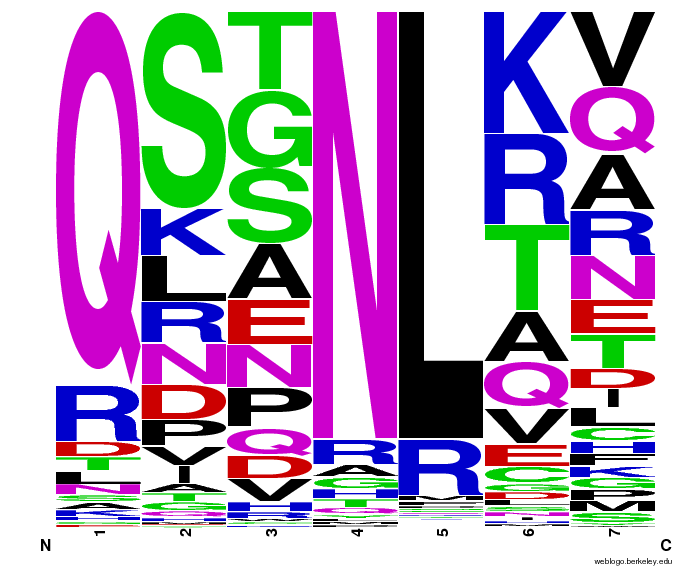
All of the sequenced colonies used the Zif268 backbone, but since the TGG codon is not unlike known Zif268-based zinc finger binding sequences, this may imply that Zif268 is the optimal backbone for such a base triplet. The actual DNA-binding helix is much more variable, and we will continue to test the strength of the binding interactions of these zinc fingers and look for trends in their amino acid make-up.
Genome-Based One-Hybrid Selection Strain
We designed a one-hybrid metabolic system that was entirely genome-based. Using multiplex automated genome engineering (MAGE) and lambda red, we knocked out HisB, PyrF, and rpoZ; inserted a kanamycin cassette-zinc finger binding site-His3-URA3 construct into the 1529620 locus; and changed the zinc finger binding site directly on the genome. The strain was fully characterized and was sensitive enough to recognize a valid zinc finger when diluted as much as one into one million of negative controls. See here for more details.
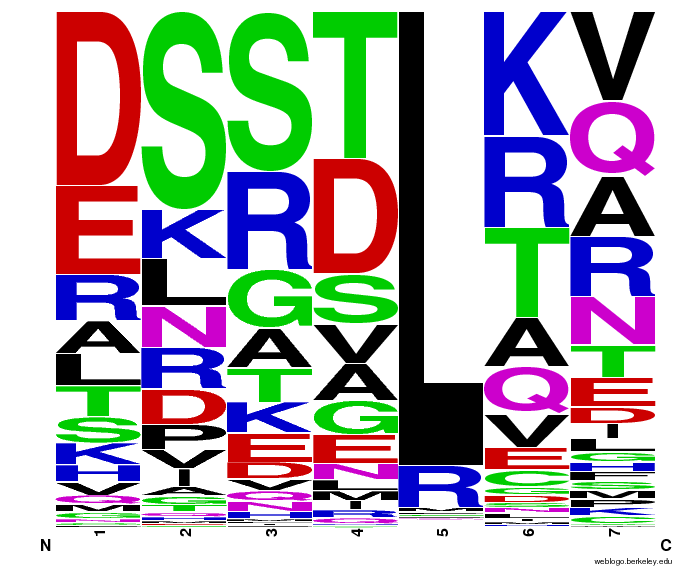
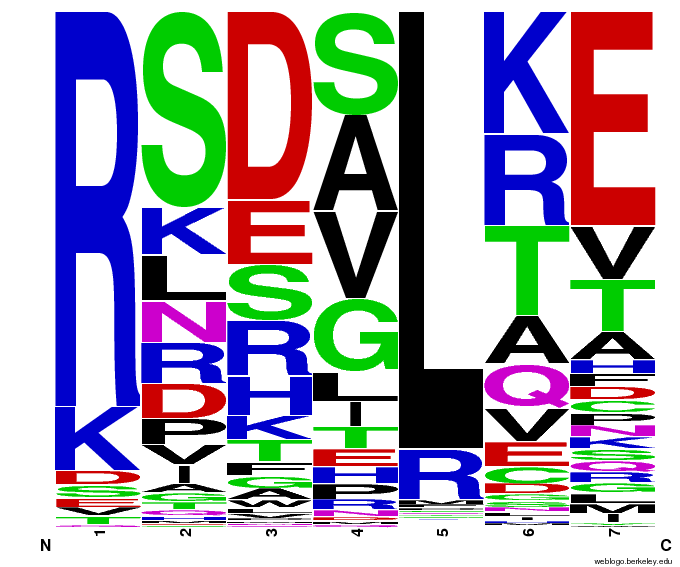
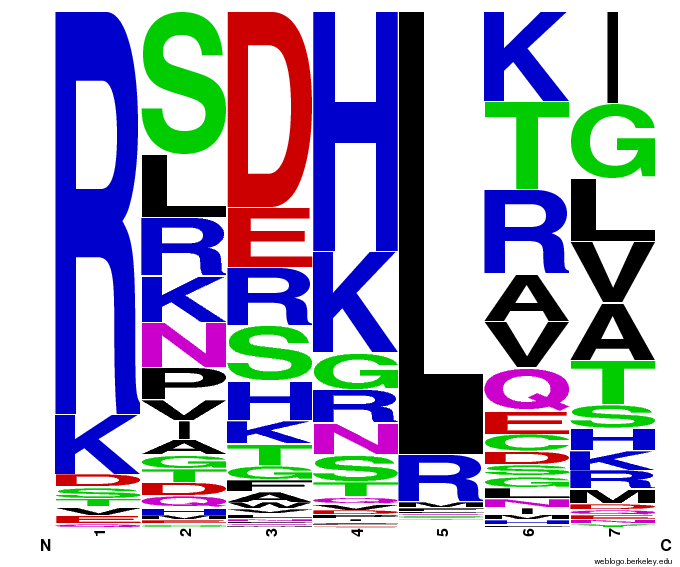
Bioinformatics: 55,000 Potential Zinc Fingers
We made 55,000 sequences, distributed evenly among 6 DNA target triplets. That's 9150 per target.
Because our program's output changes dramatically based on the input triplet, no two sets of sequences are the same:
Make your own zinc finger sequences using our [http://sourceforge.net/projects/harvardigem/ generator].
Written in Python 2.7, this program is what team members created to generate 55,000 zinc finger sequences, as described on our design page.
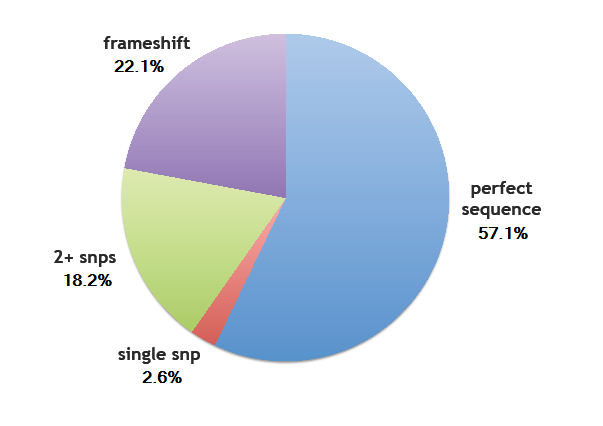
Sequencing Results of Library Transformation
Error rates
- Perfect sequence matches a designed ZF: 57.1% (44/77)
- Single SNP: 2.6% (2/77)
- Two or more SNPs: 18.2% (14/77)
- Frame shift: 22.1% (17/77)
We determined the overall per base pair error rate for this set sequenced to be around 1/200, which includes errors generated by the chip, or generated during PCR and assembly. These are a bit higher than those found by Kosuri, et al., but within a reasonable margin.
 "
"