Team:UC Davis/PromoterFamilies
From 2011.igem.org
(Difference between revisions)
Aheuckroth (Talk | contribs) (Created page with "{{Team:UC_Davis/Head}} {{Team:UC_Davis/MutantWidget}} <html> <style> #navcon { background: url('https://static.igem.org/mediawiki/2011/0/05/Ucd_Protein_banner.jpg'); } </style> <scri...") |
Aheuckroth (Talk | contribs) |
||
| Line 15: | Line 15: | ||
<html> | <html> | ||
| + | <div class="floatbox"> | ||
| + | <h1>Promoter Families</h1> | ||
| + | <div class="floatbox3"> | ||
| + | Repressible promoters are useful when complex genetic circuits. Because their activity can be modulated by the expression repressor proteins, chemical induction or other factors, they offering more control over their activity levels than constitutive promoters. We chose to expand the LacI, TetR and Lambda c1 BioBricks into part families to offer synthetic biologists a broader selection of repressible promoters to choose from. | ||
| + | |||
<div class="floatbox"> | <div class="floatbox"> | ||
<h1>LacI</h1> | <h1>LacI</h1> | ||
| Line 22: | Line 27: | ||
<p>To the left is a small render of the LacI tetramer bound to its operator.</p> | <p>To the left is a small render of the LacI tetramer bound to its operator.</p> | ||
</div> | </div> | ||
| - | |||
<div class="floatbox2"> | <div class="floatbox2"> | ||
Revision as of 17:55, 22 October 2011
Start a Family
Got a favorite BioBrick? Check our our process for expanding basic parts into part families.Criteria
View our judging criteria for iGEM 2011 here.
Promoter Families
Repressible promoters are useful when complex genetic circuits. Because their activity can be modulated by the expression repressor proteins, chemical induction or other factors, they offering more control over their activity levels than constitutive promoters. We chose to expand the LacI, TetR and Lambda c1 BioBricks into part families to offer synthetic biologists a broader selection of repressible promoters to choose from.

The above graph shows our initial mutants. We picked 87 potential mutants from transformation plates and ran them in our plate reader to quantitatively measure fluorescence. The green bars represent variants that are at least 1.5 standard deviations from the average wildtype expression level.
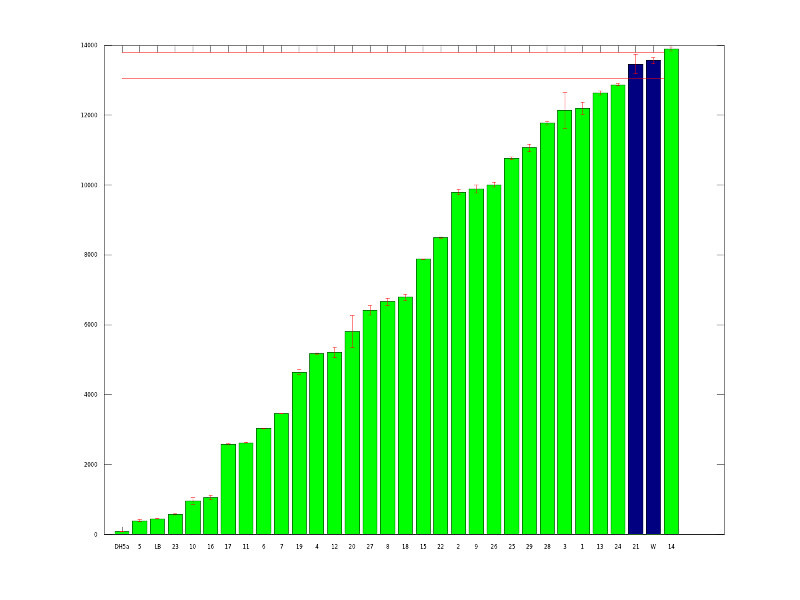
After gathering this data, we picked 29 mutants which represented a good range of expression. From there we did more fluorescence tests to obtain a final 7 mutants. After choosing our mutants, we did more rigorous characterization as outlined on our Data page.

The sequences above show our 7 LacI mutants. There are between 1 and 7 mutations in each sequence as indicated by the red bases. All 7 sequences have mutations between bases 100 and 200 which contain the known locations of the CAP binding site(bases 88-127) and LacI binding site (bases 166-200). Read more about our mutants on their Parts Registry pages.
LacI
The lac repressor is responsible for regulating the metabolism of lactose. In the absence of lactose, LacI forms a tetramer with identical subunits which appears as two dimers. Each dimer binds in the major groove of the DNA binding region which subsequently blocks the RNA polymerase from binding. In nature, allolactose will bind the repressor leading to transcription of the lac operon. Using IPTG as an inducer has the same effect as allolactose.
To the left is a small render of the LacI tetramer bound to its operator.
Mutant Screening

The above graph shows our initial mutants. We picked 87 potential mutants from transformation plates and ran them in our plate reader to quantitatively measure fluorescence. The green bars represent variants that are at least 1.5 standard deviations from the average wildtype expression level.

DNA Sequences

The sequences above show our 7 LacI mutants. There are between 1 and 7 mutations in each sequence as indicated by the red bases. All 7 sequences have mutations between bases 100 and 200 which contain the known locations of the CAP binding site(bases 88-127) and LacI binding site (bases 166-200). Read more about our mutants on their Parts Registry pages.
 "
"





