Team:HKU-Hong Kong/Results
From 2011.igem.org
| Line 9: | Line 9: | ||
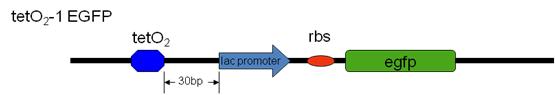
Our team have successfully inserted the tetO2-GFP sites (tetO2-0 EGFP , tetO2-0 sfGFP and tetO2-1 EGFP) into the E.Coli MG1655 genome (attTn7 site) by recombination with our designed plasmid containing the tet-O2-GFP site. | Our team have successfully inserted the tetO2-GFP sites (tetO2-0 EGFP , tetO2-0 sfGFP and tetO2-1 EGFP) into the E.Coli MG1655 genome (attTn7 site) by recombination with our designed plasmid containing the tet-O2-GFP site. | ||
Subsequently, we transformed our silencer plasmid in to these tetO2-GP strains and the GFP intensity is measured. | Subsequently, we transformed our silencer plasmid in to these tetO2-GP strains and the GFP intensity is measured. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 49: | Line 38: | ||
|- | |- | ||
|style="width:900px;"| | |style="width:900px;"| | ||
| + | |||
| + | |||
| + | |||
| + | [EGFP: Enhanced Green Fluorescent Protein, sfGFP: Superfolder Green Fluorescent protein ] | ||
| + | |||
| + | |||
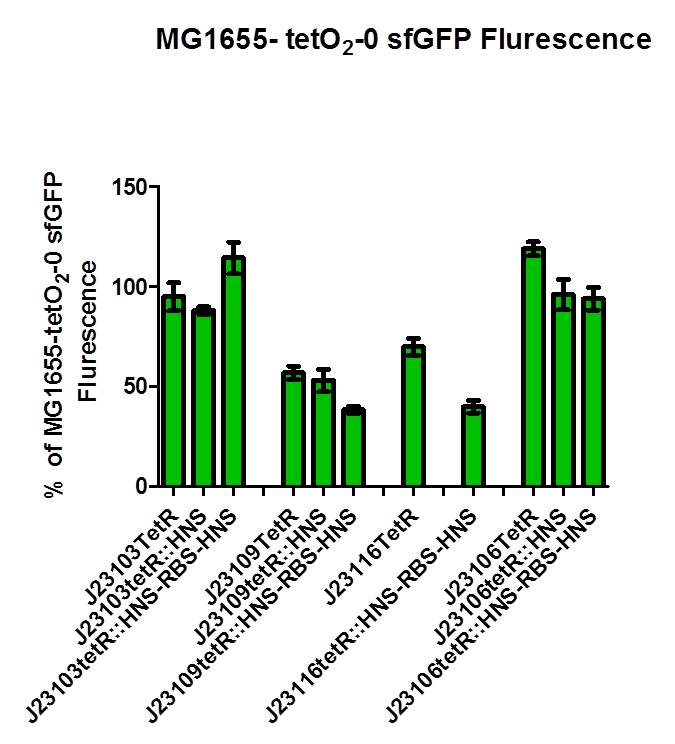
| + | The GFP intensity results are as follow: | ||
| + | |||
| + | |||
| + | The untransformed E.Coli MG1655 (tetO2-0 EGFP, tetO2-0 sfGFP or tetO2-1 EGFP incorporated into the genome) is set as control and the fluorescence intensity is calibrated as 100%. | ||
| + | |||
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
Latest revision as of 19:29, 5 October 2011
| Results |
| GFP intensity |
|
Our team have successfully inserted the tetO2-GFP sites (tetO2-0 EGFP , tetO2-0 sfGFP and tetO2-1 EGFP) into the E.Coli MG1655 genome (attTn7 site) by recombination with our designed plasmid containing the tet-O2-GFP site. Subsequently, we transformed our silencer plasmid in to these tetO2-GP strains and the GFP intensity is measured.
|
|
|
|
[EGFP: Enhanced Green Fluorescent Protein, sfGFP: Superfolder Green Fluorescent protein ]
|
|
|
|
As shown in above figures, when using promoters J23109, J23116, J23106, there is a subsequent lower flurescence intensity detected. When only tetR is produced, there is already some repression. But the use of our fusion protein (tetR-HNS), the fluorescence is even lowered. This provides an insight for us that our designed repression system could be working. When we also expressed the native H-NS in addition to the bacteria own H-NS, the fluorescence intensity is the lowest. It can be reasoned that when our fusion protein tetR-HNS bind to the tet-O sites, the chance for oligomerization and hence repression is increased as there are more native H-NS available.
|
|
From the above result, in different promoter driven fusion tetR-HNS proteins system, their repressions are different. For example, when using J23103 promoter, all designed repressor and also the tetR have higher fluorescence intensity than the control. As for J23109, there is lower fluorescence intensity in tetR-HNS but higher intensity when native HNS is also expressed. In other two promoters, the results are also unexpected. It seems that tetO-1 site is not suitable when the tetR recognition site is in too close proximity with the pLac promoter and the EGFP gene.
|
| Gel shift assay |
|
Due to failures in our experiment and problem of time constraints, our team failed to complete the gel shift assay to detect the presence of DNA-Protein interaction between our fusion protein and the recognition sites. |
 "
"