Team:HKU-Hong Kong/Lab Diaries
From 2011.igem.org
(Difference between revisions)
| Line 56: | Line 56: | ||
</OL> | </OL> | ||
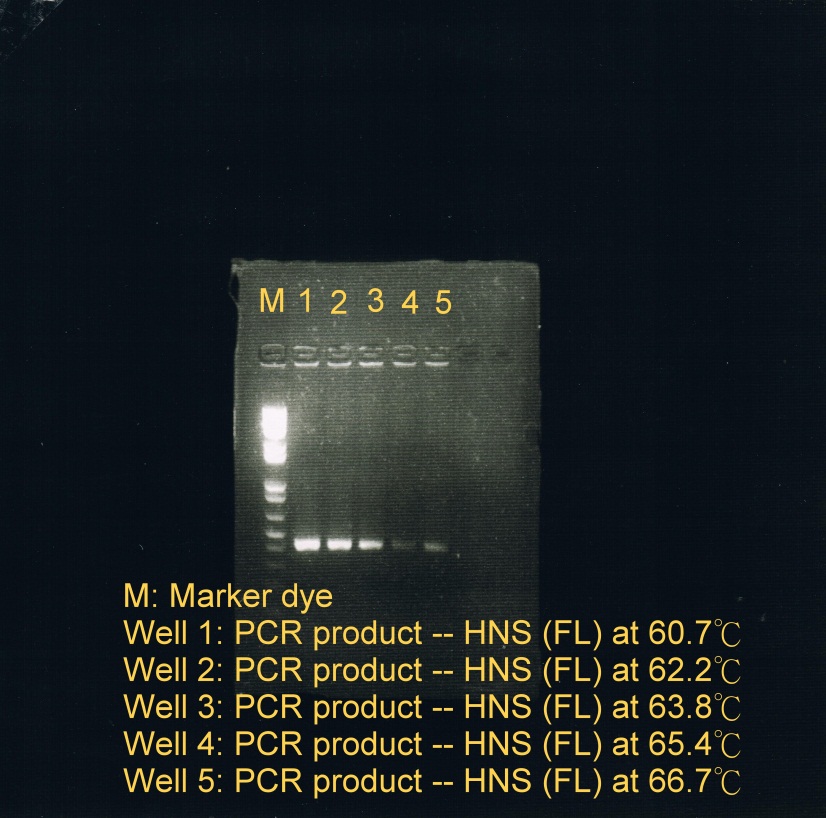
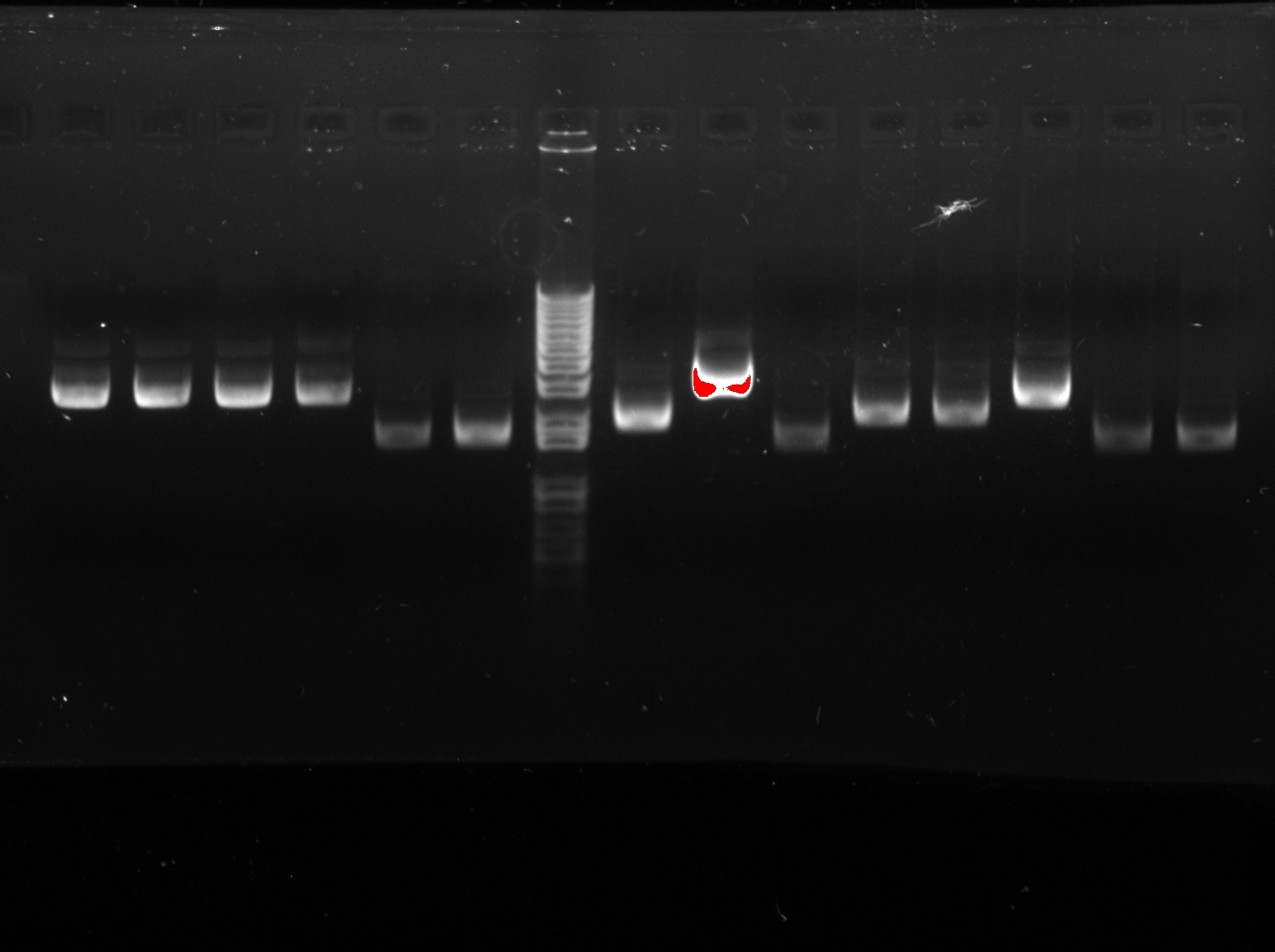
<LI>Electrophoresis was carried to determine which temperature best suit the annealing phase. From the gel image, it was observed that a high concentration of products was resulted at annealing temperature between 60.7C and 62.2C. | <LI>Electrophoresis was carried to determine which temperature best suit the annealing phase. From the gel image, it was observed that a high concentration of products was resulted at annealing temperature between 60.7C and 62.2C. | ||
| + | |||
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| Line 64: | Line 65: | ||
|} | |} | ||
</div> | </div> | ||
| - | |||
|- | |- | ||
|style="width:900px;"|'''Week 4-5''' | |style="width:900px;"|'''Week 4-5''' | ||
| Line 94: | Line 94: | ||
<OL> | <OL> | ||
<LI>The sticky ends of the enzyme products was transformed into blunt ends using PCR. | <LI>The sticky ends of the enzyme products was transformed into blunt ends using PCR. | ||
| - | |||
|- | |- | ||
|style="width:900px;"|'''Week 8''' | |style="width:900px;"|'''Week 8''' | ||
| Line 111: | Line 110: | ||
<OL> | <OL> | ||
<LI>0.5uL of CIP alkaline phosphatase was added to prevent self-ligation to each enzyme digest reaction mixture. | <LI>0.5uL of CIP alkaline phosphatase was added to prevent self-ligation to each enzyme digest reaction mixture. | ||
| - | |||
|- | |- | ||
|style="width:900px;"|'''Week 9''' | |style="width:900px;"|'''Week 9''' | ||
| Line 120: | Line 118: | ||
</OL> | </OL> | ||
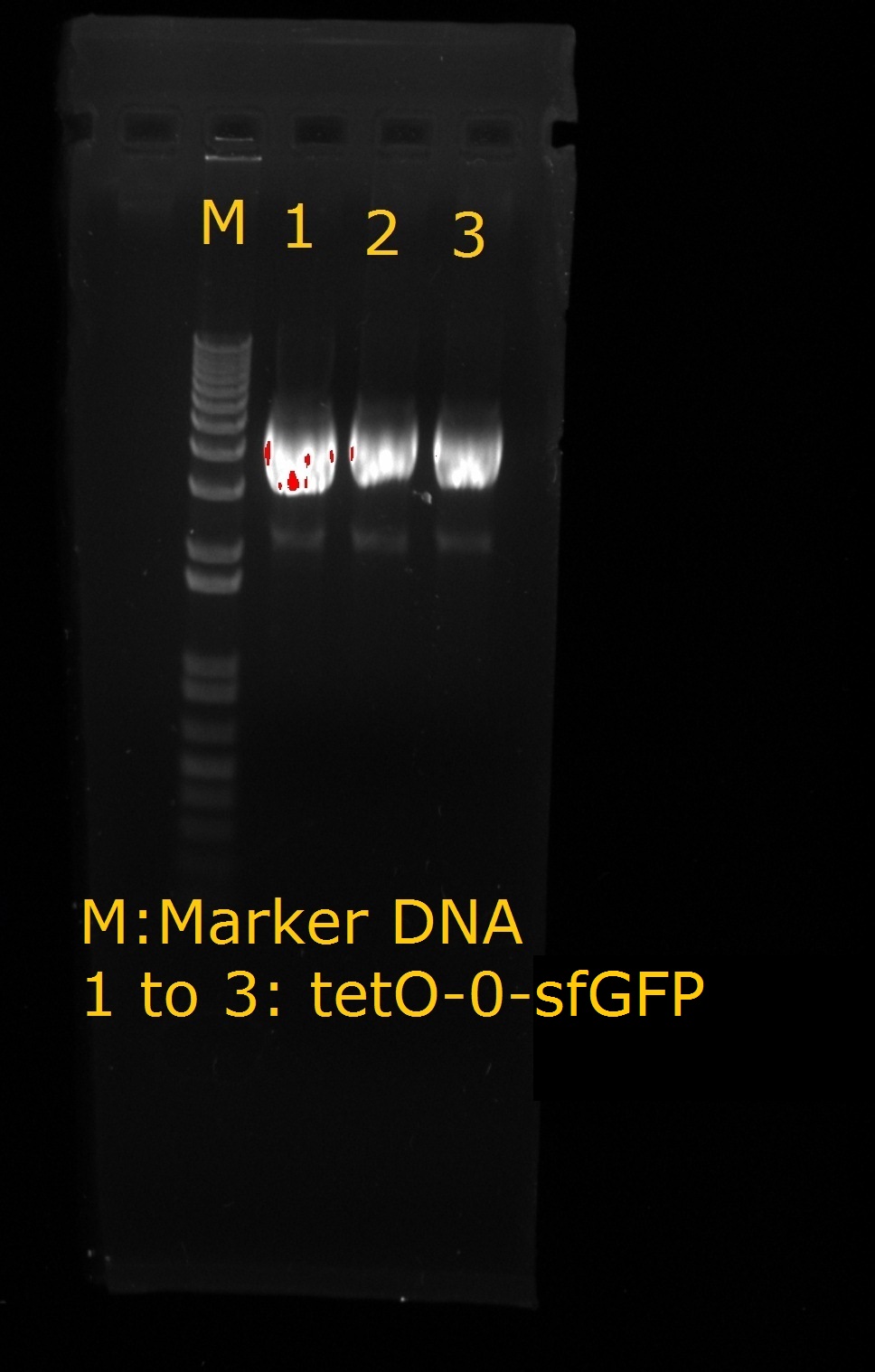
<LI>Production of tetO2 – 0 –sfGFP | <LI>Production of tetO2 – 0 –sfGFP | ||
| + | |||
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| Line 167: | Line 166: | ||
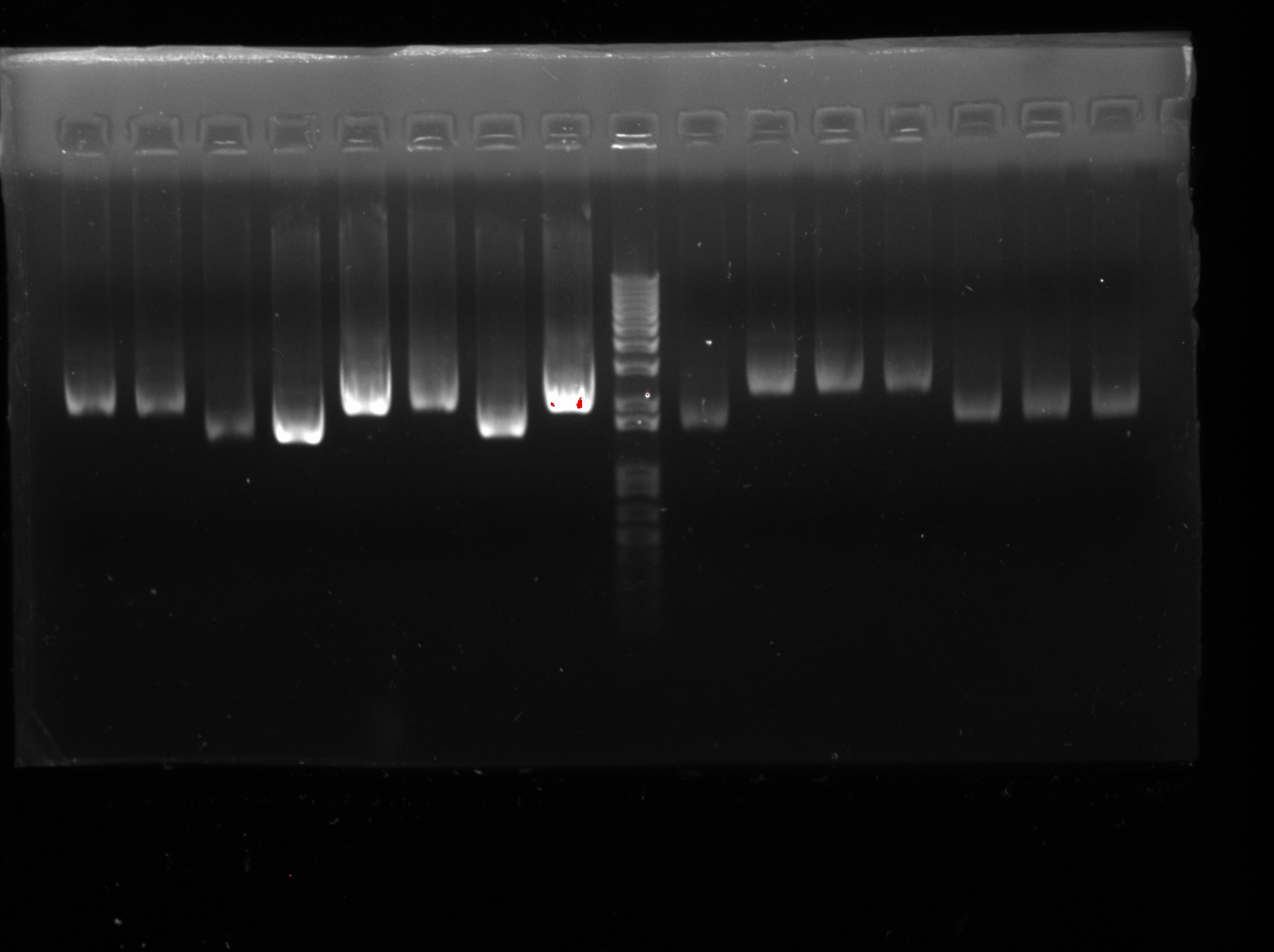
<LI>pSB1C3-HNS | <LI>pSB1C3-HNS | ||
<LI>Electrophoresis was carried out to confirm the success of extraction) (except J23116RH) | <LI>Electrophoresis was carried out to confirm the success of extraction) (except J23116RH) | ||
| + | |||
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| Line 175: | Line 175: | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
<div ALIGN=CENTER> | <div ALIGN=CENTER> | ||
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
Latest revision as of 06:59, 5 October 2011
| Lab Diaries |
| Week 1
Transformation of reporter DNA (pEGFP-loxp-km-loxp) into DH10B (non-virulent strain E. coli) with antibiotic resistance (Chloramphenicol – Cm) |
Week 2
|
Week 3
|
| Week 4-5
tetO2 – 0 and tetO2 – 4
|
| Week 6
Overlap PCR was carried out to produce fusion protein gene
|
| Week 7
Overlap PCR was carried out to produce fusion protein gene tetR::HNS (2-90)
|
Week 8
|
Week 9
|
Week 10
|
Week 11
|
Week 12
|
Week 13
|
Week 14
|
 "
"