Team:USTC-China/Protocol
From 2011.igem.org
(→Artificial Innate Immunity System Construction and Testing) |
(→Artificial Innate Immunity System Construction and Testing) |
||
| Line 35: | Line 35: | ||
==Artificial Innate Immunity System Construction and Testing== | ==Artificial Innate Immunity System Construction and Testing== | ||
| - | <p> For the simulation, a cassete containing a ColE7-ImmE7 complex gene([http://partsregistry.org/Part:BBa_K117001 | + | <p> For the simulation, a cassete containing a ColE7-ImmE7 complex gene([http://partsregistry.org/Part:BBa_K117001 BBa_K117001] ), Toggle Switch-Aptamer-cheZ Device, and Lysis gene([BBa_K117000]http://partsregistry.org/Part:BBa_K117000) was assembled to create a destruction module. Then using this module to transform the E.coli RP1616 competence containing the LuxPR-cI Device. After transformation, diluted engineering bacteria and target bacteria suspensions from mid-log-phaase cultures were applied to the sites(as Figure3 shown) of the Semi-Solid Media. The plates were dried the room temperature at for 15 min, and incubated at 37℃ for 10h to observe the form change of two colonies.</p> |
[[File:C().jpg|center| Figure3.(Colony1: engineering bacteria, Colony2: target bacteria)]] | [[File:C().jpg|center| Figure3.(Colony1: engineering bacteria, Colony2: target bacteria)]] | ||
<p align=center>Figure3.(Colony1: engineering bacteria, Colony2: target bacteria)</p> | <p align=center>Figure3.(Colony1: engineering bacteria, Colony2: target bacteria)</p> | ||
Revision as of 12:23, 2 October 2011
Aptamer-cheZ Device Construction
The cheZ gene was cloned from E.coli TOP10 strain using PCR. A cassete containing a theophylline-sensitive aptamer, and the cheZ gene was assembled using PCR and was subcloned into the SpeI and PstI sites of pSB1A2 containing the lac promoter.
Clone Primer:
Forward:5'-GTTTCGAATTCGCGGCCGCTTCTAGATGCAACCATCAATCAAACC-3'
Reverse:5'-GTTTCCTGCAGCGGCCGCTACTAGTATTATTAAAATCCAAGTCTATCCAACAAATCGT-3'
Assemble Primer:
Forward:5'-GTTTCGAATTCGCGGCCGCTTCTAGGGTGATACCAGCATCGTCTTGATGCCCTTGGCAGCACCCCGCTGCAAGACAACAAG ATGCAACCATCAATCAAACC-3'
Reverse:5'-GTTTCCTGCAGCGGCCGCTACTAGTATTATTAAAATCCAAGACTATCCAACAAATCGT-3'
Assemble condition: 96°C 10min, (96°C 30s, 56°C 30s, 72°C 1min)16 cycles and each cycle the annealing temperature increase 1°C, (96°C 30s, 72°C 30s, 72°C 1min)20 cycles,72°C 10min and holding at 4°C.
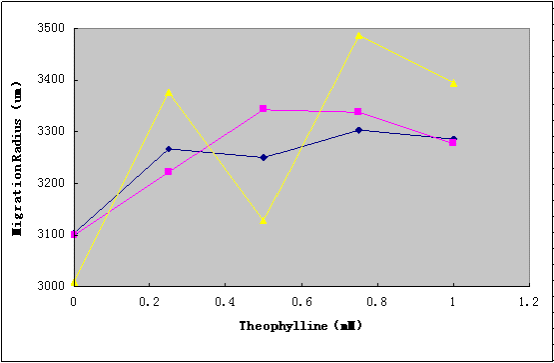
Dose-Dependent Migration Ability of Aptamer-cheZ Device
To test the migration ability, selective media (tryptone broth with different ratio of agar(0.25%, 0.3%, 0.4%), 50μg/mL ampicllin, and various concentrations of theophylline(0mM, 0.25mM, 0.5mM, 0.5mM, 0.75mM, 1mM)) was prepared in Petri dishes(85mm dia). Diluted cell suspensions from mid-log-phase cultures were applied to the center of the plates, which were dried at room temperature at for 15 min, and incubated at 37°C for 10h, and the migration radii were determined by measuring the diameter of the outermost ring of growth.
(a)
(b)
(c)
Figure1.((a)0.25%agar, (b)0.3%agar, (c)0.4%agar)
The results(Figure1) show that the Aptamer-cheZ Device is functional, and the 0.3%agar is the best ratio for the migration experiments.
Toggle Switch Device Testing and Modifying
First, test the function of the original Toggle Switch Device from PKU. After the transformation, the ratio between the colonies with red fluorescent and the colonies with green fluorescent is 8:25.
Then, modify the prototype of the Toggle Switch Device from PKU. The cI gene was subcloned into the SpeI and PstI sites of pSB3t5, a low copy plasmid, containing the LuxPR promoter to create LuxPR-cI Device. After the transformation, prepare the E.coli RP1616 competence containing the LuxPR-cI Device, then use the original Toggle Switch Device to transform this competence.
After the transformation, the ratio between the colonies with red fluorescent and the colonies with green fluorescent is 6:1.
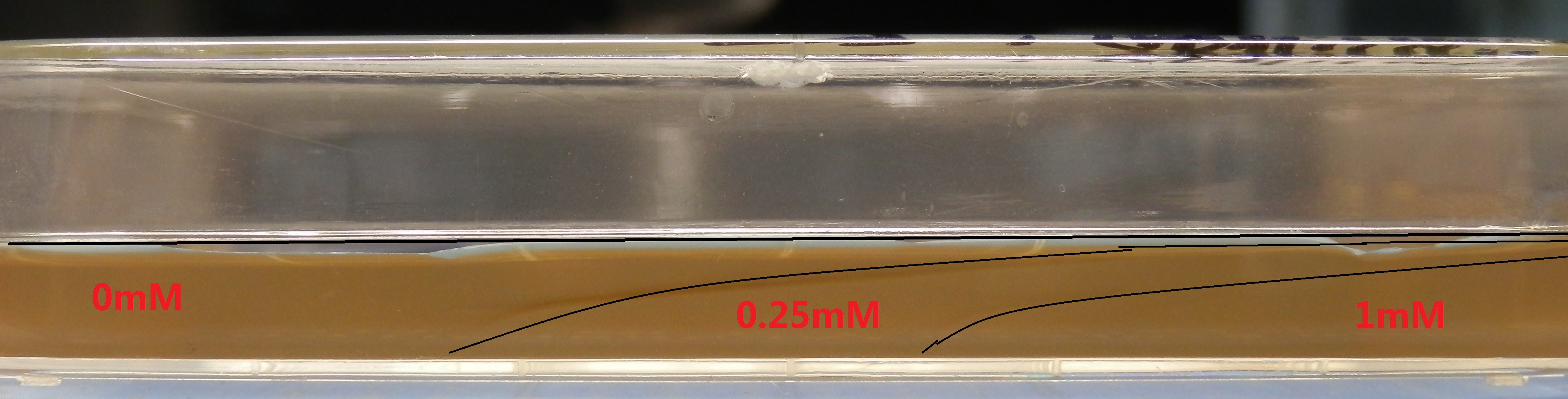
Semi-Solid Media with the Gadient of Theophylline
Media was prepared in 100mm square Petri dishes. Layers(15ml) of selective 0.3% agar containing theophylline(1mM, 0.25mM, 0mM) were poured in the pattern shown in Figure 2.
Figure2.
Each layer would solidify for 50 min before applying the following layer. After all layers were applied, the media should equilibrate at room temperature for 3.0 h.
Toggle Switch-Aptamer-cheZ Device Construction and Testing
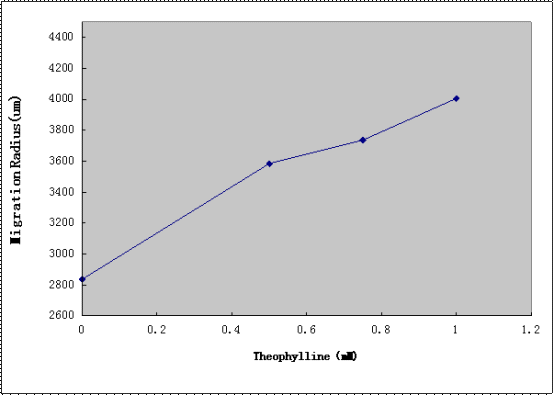
The Aptamer-cheZ Device(without lac promoter) was subcloned into the SpeI and PstI sites of the Toggle Switch Device([http://partsregistry.org/Part:BBa_K228003 BBa_K228003]) from PKU to create the Toggle Switch -Aptamer-cheZ Device. After the transformation, diluted cell suspensions from mid-log-phaase cultures were applied to the center of the Semi-Solid Media(as Figure2 shown). The plates were dried the room temperature at for 15 min, and incubated at 37℃ for 10h, and the migration radii were determined by measuring the diameter of the outermost ring of growth.
Toggle Switch-Aptamer-cheZ Device(modified) Construction and Testing
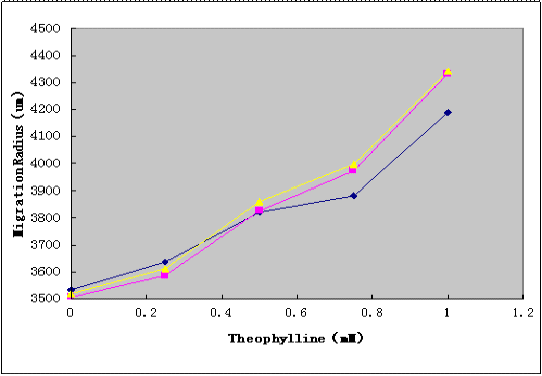
Using Toggle Switch-Aptamer-cheZ Device to transform the E.coli RP1616 competence containing the LuxPR-cI Device. After transformation, diluted cell suspensions from mid-log-phaase cultures were applied to the center of the Semi-Solid Media(as Figure2 shown). The plates were dried the room temperature at for 15 min, and incubated at 37℃ for 10h, and the migration radii were determined by measuring the diameter of the outermost ring of growth.
Artificial Innate Immunity System Construction and Testing
For the simulation, a cassete containing a ColE7-ImmE7 complex gene([http://partsregistry.org/Part:BBa_K117001 BBa_K117001] ), Toggle Switch-Aptamer-cheZ Device, and Lysis gene([BBa_K117000]http://partsregistry.org/Part:BBa_K117000) was assembled to create a destruction module. Then using this module to transform the E.coli RP1616 competence containing the LuxPR-cI Device. After transformation, diluted engineering bacteria and target bacteria suspensions from mid-log-phaase cultures were applied to the sites(as Figure3 shown) of the Semi-Solid Media. The plates were dried the room temperature at for 15 min, and incubated at 37℃ for 10h to observe the form change of two colonies.
Figure3.(Colony1: engineering bacteria, Colony2: target bacteria)
 "
"