Team:HKU-Hong Kong/Modelling
From 2011.igem.org
(Difference between revisions)
| Line 7: | Line 7: | ||
|style="width:900px;"| | |style="width:900px;"| | ||
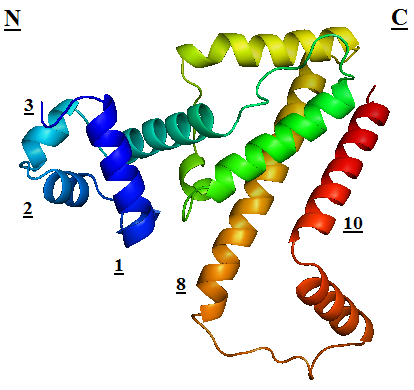
TetR regulates the most abundant resistance mechanism against the antibiotic tetracycline in gram-negative bacteria. It consists of 208 residues, being folded into a 10 α – helices. α1 – α3 forms the DNA-binding domain, within which α2 – α3 constitute the classical helix-turn-helix motifs. α5 – α10 forms the binding domain of the tetracycline – magnesium complex, [MgTc]+, α5, α8 and α10 are helices of the rigid scaffold, within which α8 and α10 form the central part of the regulatory domain with the dyad related α8’ and α10’. α4 acts as the hydrophobic center of the DNA binding domain, and links the DNA-binding domain to the regulatory domain.<br/> | TetR regulates the most abundant resistance mechanism against the antibiotic tetracycline in gram-negative bacteria. It consists of 208 residues, being folded into a 10 α – helices. α1 – α3 forms the DNA-binding domain, within which α2 – α3 constitute the classical helix-turn-helix motifs. α5 – α10 forms the binding domain of the tetracycline – magnesium complex, [MgTc]+, α5, α8 and α10 are helices of the rigid scaffold, within which α8 and α10 form the central part of the regulatory domain with the dyad related α8’ and α10’. α4 acts as the hydrophobic center of the DNA binding domain, and links the DNA-binding domain to the regulatory domain.<br/> | ||
| - | <div ALIGN= | + | <div ALIGN=CENTER> |
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
|- | |- | ||
| Line 20: | Line 20: | ||
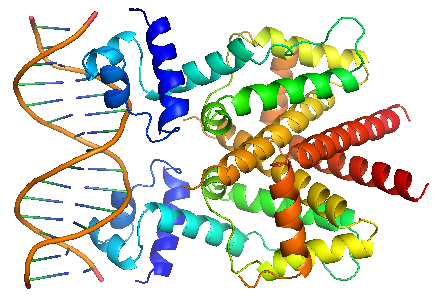
In the presence of tetracycline – magnesium complex, [MgTc]+, residue His100 and Thr103 of helix α6 are displaced, causing the shifting of helix α6 towards the C terminal. Both C and N terminal of α4 shift because of the movement of α6 and the formation of hydrogen bond between α4 and [MgTc]+. α3 is displaced as a consequence, which releases the bonding between tetR and tetO.<br/> | In the presence of tetracycline – magnesium complex, [MgTc]+, residue His100 and Thr103 of helix α6 are displaced, causing the shifting of helix α6 towards the C terminal. Both C and N terminal of α4 shift because of the movement of α6 and the formation of hydrogen bond between α4 and [MgTc]+. α3 is displaced as a consequence, which releases the bonding between tetR and tetO.<br/> | ||
| - | <div ALIGN= | + | <div ALIGN=CENTER> |
{| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
|- | |- | ||
|[[Image:TetR-tetO.png|250px]] | |[[Image:TetR-tetO.png|250px]] | ||
|- | |- | ||
| - | |TetR | + | |TetR binding with TetO |
|} | |} | ||
</div> | </div> | ||
|} | |} | ||
Revision as of 11:25, 30 September 2011
| Modelling |
| The tetracycline inducible Tet repressor-operator system |
|
TetR regulates the most abundant resistance mechanism against the antibiotic tetracycline in gram-negative bacteria. It consists of 208 residues, being folded into a 10 α – helices. α1 – α3 forms the DNA-binding domain, within which α2 – α3 constitute the classical helix-turn-helix motifs. α5 – α10 forms the binding domain of the tetracycline – magnesium complex, [MgTc]+, α5, α8 and α10 are helices of the rigid scaffold, within which α8 and α10 form the central part of the regulatory domain with the dyad related α8’ and α10’. α4 acts as the hydrophobic center of the DNA binding domain, and links the DNA-binding domain to the regulatory domain. |
 "
"