Team:Washington/Celiacs/Results
From 2011.igem.org
(→Purifying and characterizing promising mutants for accurate rate comparison) |
|||
| Line 28: | Line 28: | ||
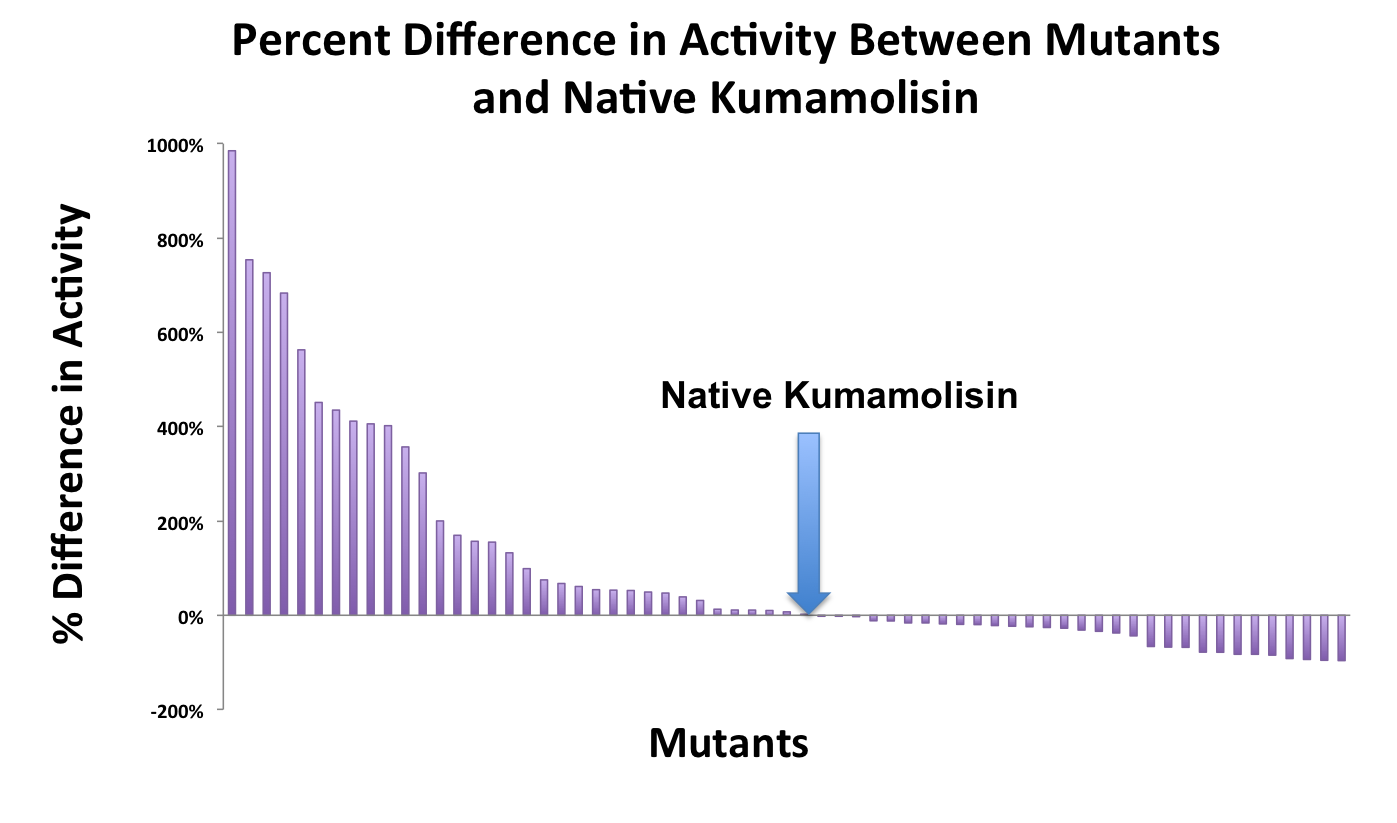
[[File:Washington Mutant Screen Percent IncDec.jpg|center|800px|thumb|Over 100 unique mutants were screened with a whole cell lysate assay for improved activity on the PQLP model substrate.]] | [[File:Washington Mutant Screen Percent IncDec.jpg|center|800px|thumb|Over 100 unique mutants were screened with a whole cell lysate assay for improved activity on the PQLP model substrate.]] | ||
| - | ==Purifying and characterizing promising mutants for accurate rate comparison== | + | =='''Purifying and characterizing promising mutants for accurate rate comparison'''== |
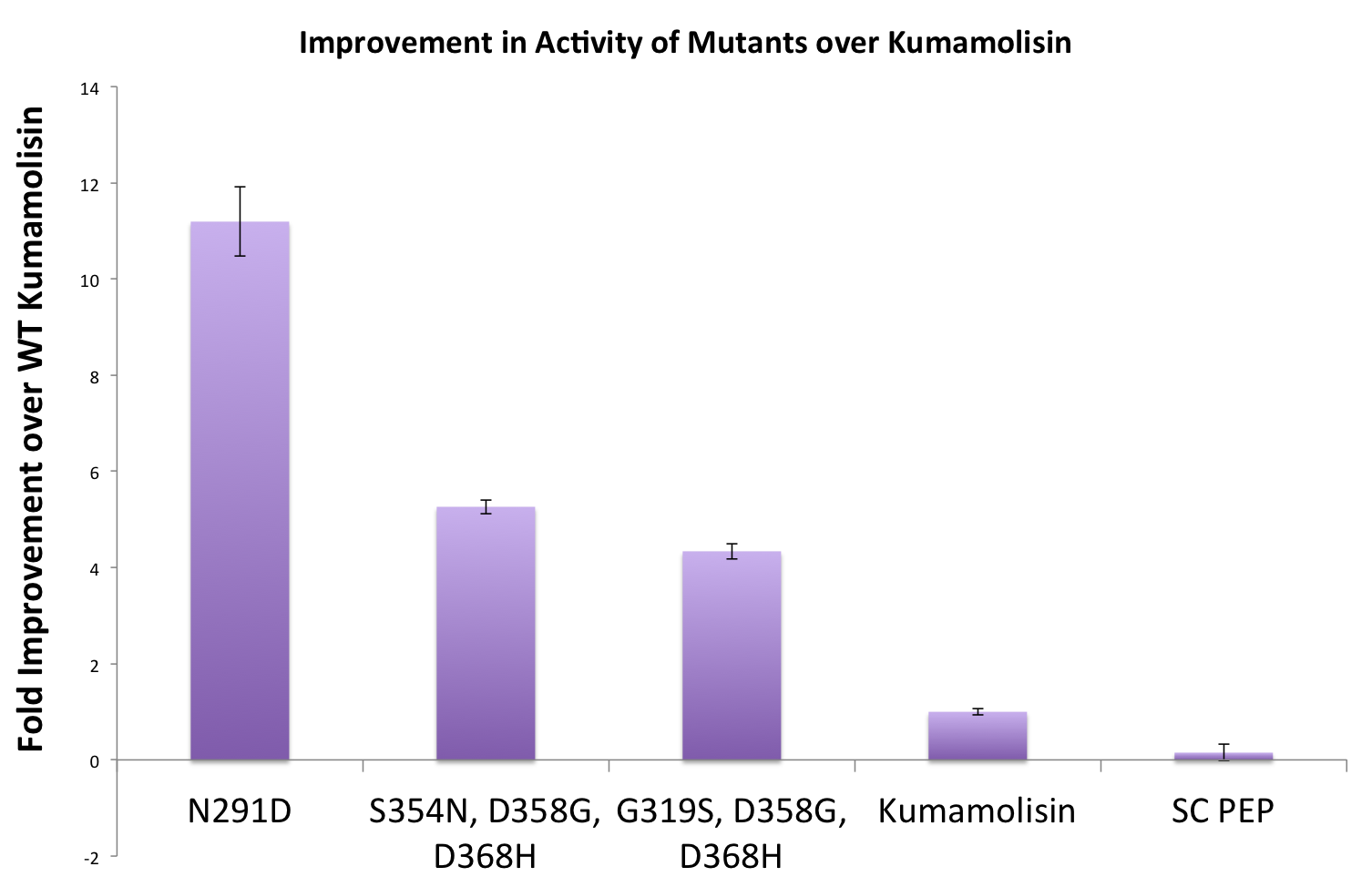
Once we had identified mutants that showed a promising increase in activity from the wild-type kumamolisin, we purified and characterized activity in concentration controlled fluorescence assays, identical to the fluorescence system used for the whole cell lysate assay. Our best mutant demonstrated an 11-fold increase in activity from the native enzyme. | Once we had identified mutants that showed a promising increase in activity from the wild-type kumamolisin, we purified and characterized activity in concentration controlled fluorescence assays, identical to the fluorescence system used for the whole cell lysate assay. Our best mutant demonstrated an 11-fold increase in activity from the native enzyme. | ||
| Line 37: | Line 37: | ||
---- | ---- | ||
| - | |||
| - | |||
=Combining Mutants for the construction of a Gluten Hydrolase= | =Combining Mutants for the construction of a Gluten Hydrolase= | ||
Revision as of 02:35, 23 September 2011
Testing Kumamolisin-As against SC-PEP
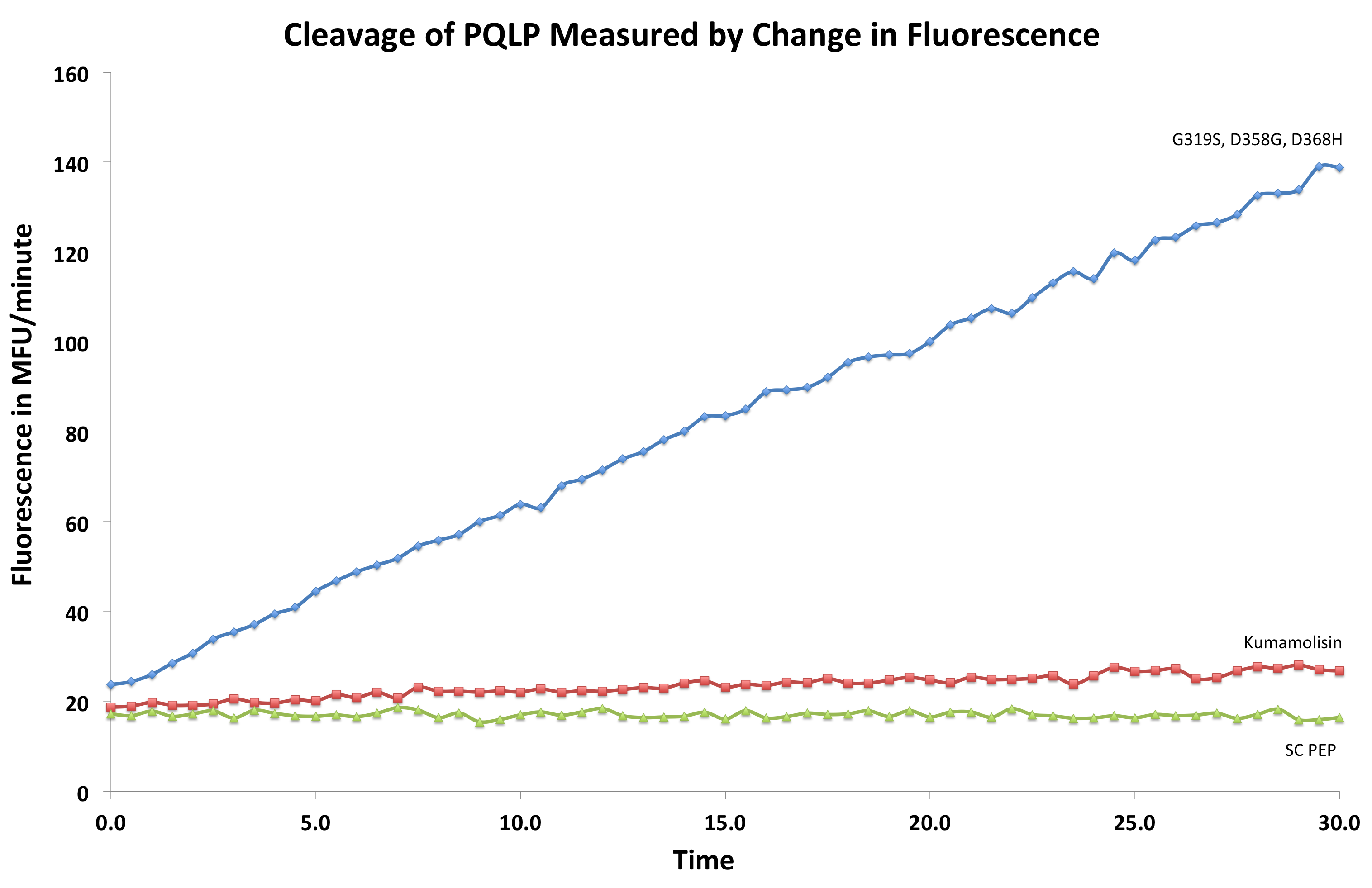
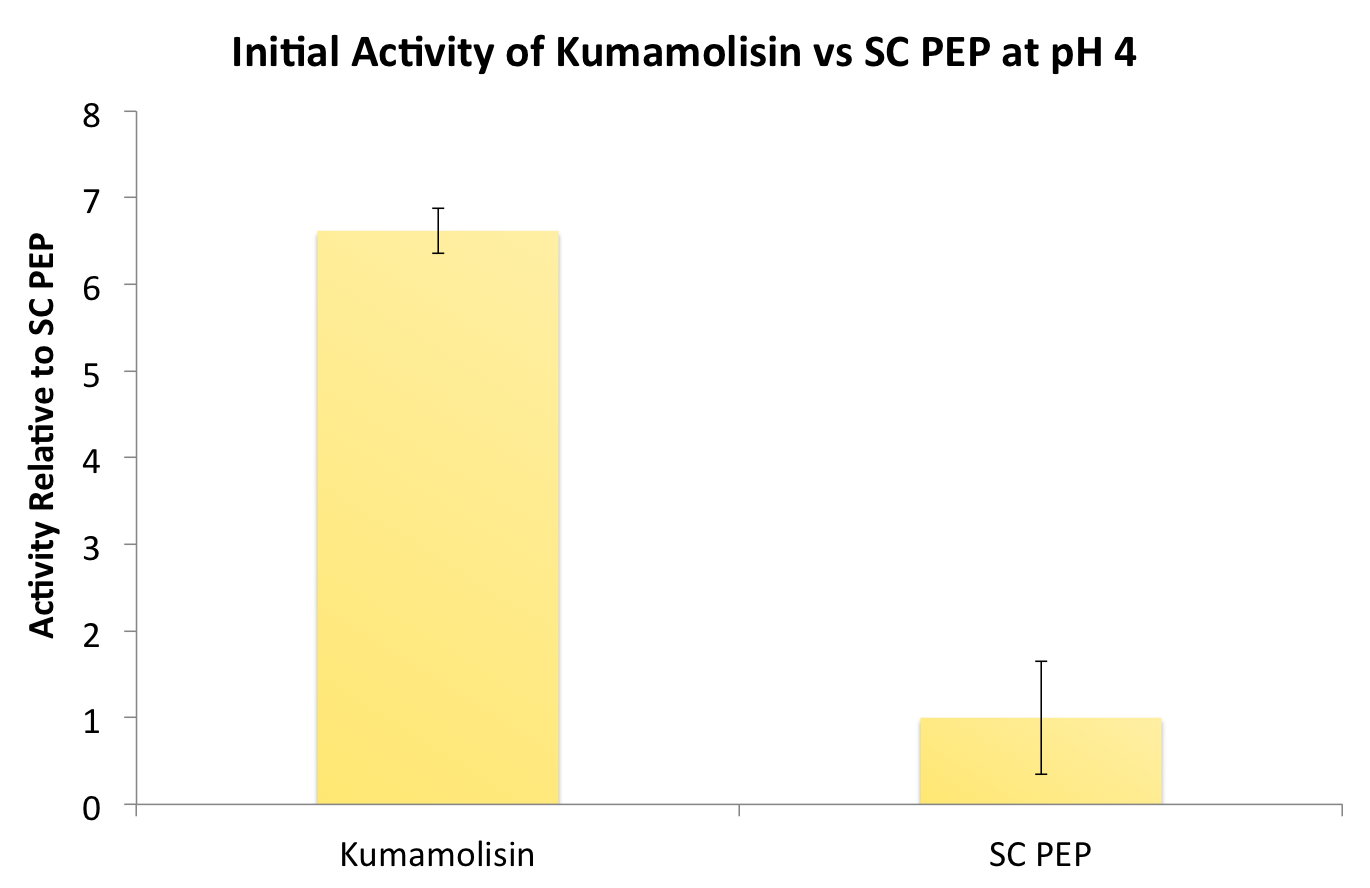
After finding Kumamolisin-As to be the ideal enzyme for our purposes, we used the assay described below to test it against SC-PEP to determine comparative activity levels, resulting in evidence that wild-type Kumamolisin-As is already over 7 fold better than SC-PEP.
Testing mutants for activity on breaking down PQLP
Using a whole cell lysate assay to screen a large number of mutants for good activity
In order to determine whether our proposed mutations to the wild-type Kumamolisin improved the ability of the enzyme to break down PQLP, we tested each mutant with a whole cell lysate fluorescence assay. Cells harboring the expressed mutants were lysed and the assay was performed at pH 4, mimicking the gastric environment. The released enzymes, after being roughly separated from cell material, were added to a fluorescent PQLP that had been conjugated to a quencher. Thus, no fluorescence was achieved until the peptide had been cleaved and the fluorophore had been released from the quencher. This allowed a relative assessment of rate of enzyme activity by measuring increase in fluorescence of the system.
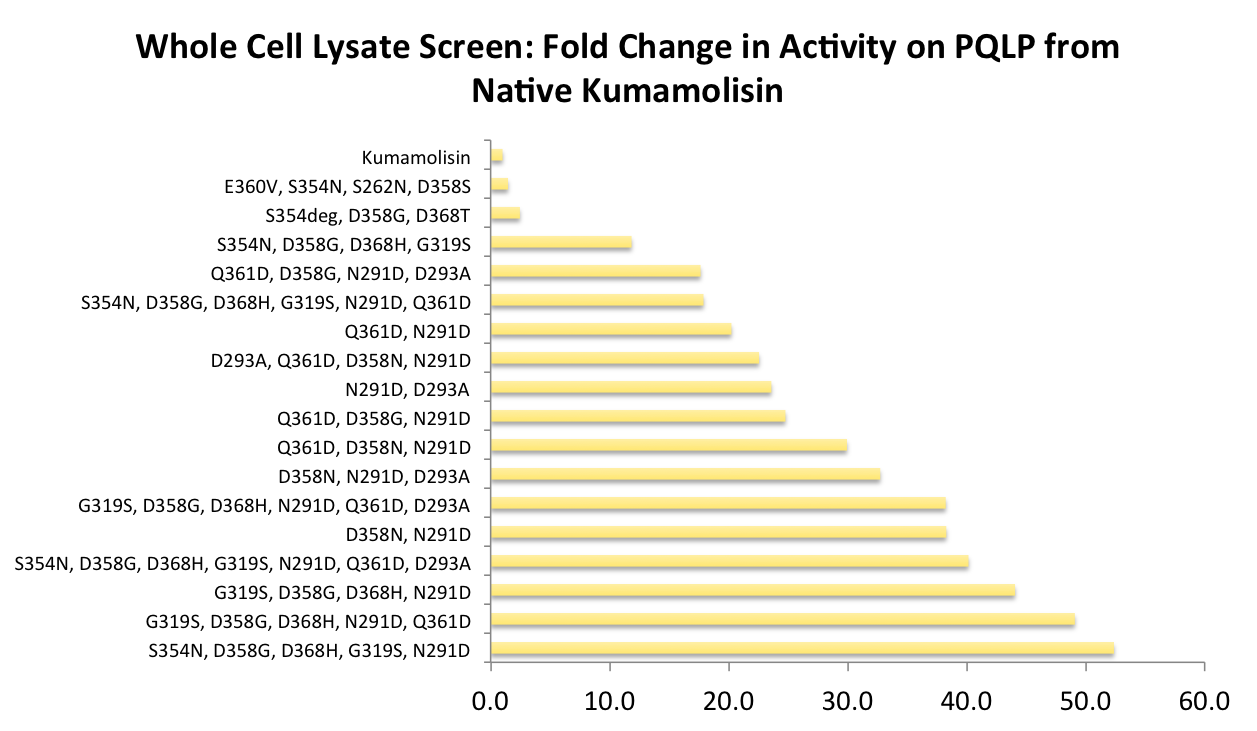
As one might expect, our first screen of mutants showed some mutants with a decrease in activity from the wild-type, some showed no change, and some actually showed great increase in activity. One single point mutant showed close to a 1000% increase in activity from wild-type Kumamolisin!
Purifying and characterizing promising mutants for accurate rate comparison
Once we had identified mutants that showed a promising increase in activity from the wild-type kumamolisin, we purified and characterized activity in concentration controlled fluorescence assays, identical to the fluorescence system used for the whole cell lysate assay. Our best mutant demonstrated an 11-fold increase in activity from the native enzyme.
Combining Mutants for the construction of a Gluten Hydrolase
A second library of based on the first round of mutagensis was constructed and tested
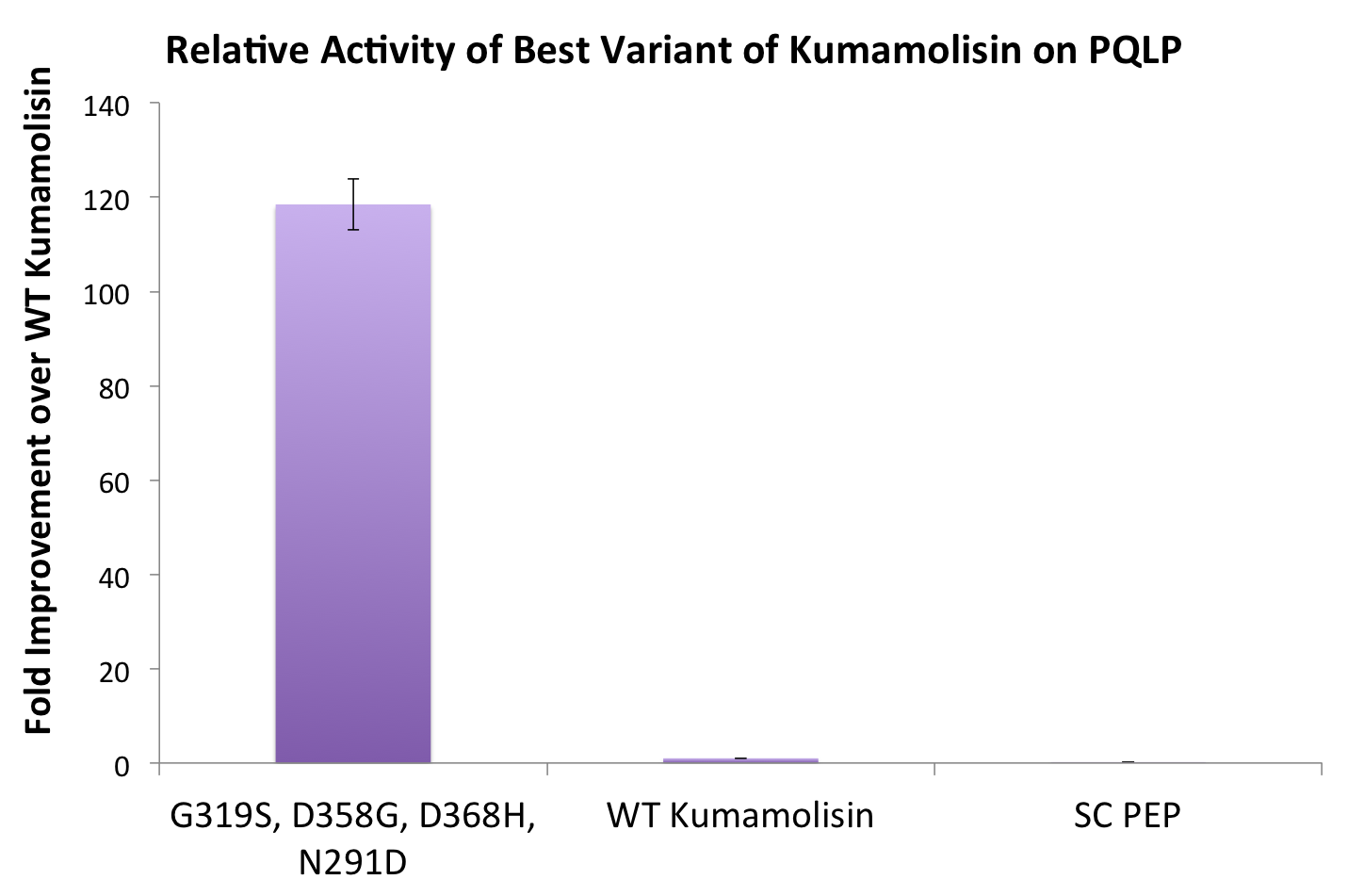
One of the combinatorial mutants resulted in over a 100-fold increase in activity
In order to achieve even more rate improvement from the native, we repeated our mutagenesis, this time taking successful mutations and adding them all to make combinatorial variants. By combining two of our top groups of mutations from the first round, we achieved an over 100-fold increase in activity on breaking down PQLP from the wild-type enzyme. This variant enzyme is ultimately 784 times better at breaking down PQLP than SC PEP, the enzyme currently in clinical trials for treating gluten intolerance!
 "
"