Team:Debrecen Hungary/Arsenic
From 2011.igem.org
(→Background) |
(→Background) |
||
| Line 12: | Line 12: | ||
As the Edinburgh team tested the system only on distilled water samples spiked with sodium-arsenate, we contacted the team and offered our help to test the machine on real-world samples to prove that it works not only on artificial but on natural samples as well (with many different compounds that may interfere with the part's action). We also sent them twenty sterilized samples collected by a Local Public Health Organisation from different wells - this organisation has also provided data on arsenic concentrations gained from flame atomic absorption spectrometry measurements. | As the Edinburgh team tested the system only on distilled water samples spiked with sodium-arsenate, we contacted the team and offered our help to test the machine on real-world samples to prove that it works not only on artificial but on natural samples as well (with many different compounds that may interfere with the part's action). We also sent them twenty sterilized samples collected by a Local Public Health Organisation from different wells - this organisation has also provided data on arsenic concentrations gained from flame atomic absorption spectrometry measurements. | ||
| + | |||
| + | |||
| + | === Method of testing === | ||
| + | |||
| + | The contact person of Team Edinburgh 2006 sent us the protocol should be used according to their experiments. | ||
| + | We collected samples from two wells which were known to contain elevated arsenic concentration (Szegfű and Madách street, Békéscsaba, Hungary). Find these places using Google Maps: [http://maps.google.com/maps?f=q&source=s_q&hl=hu&q=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&sll=37.09024,-96.503906&sspn=34.945679,92.285156&ie=UTF8&cd=1&geocode=FeAfyAIdRVlBAQ&split=0&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ll=46.666579,21.059632&spn=0.021204,0.044546&t=h&z=15], [http://maps.google.com/maps?f=q&source=s_q&hl=hu&geocode=&q=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&sll=46.666579,21.059632&sspn=0.021204,0.044546&g=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ie=UTF8&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&ll=46.666314,21.070104&spn=0.021204,0.044546&t=h&z=15] | ||
| + | |||
| + | We used DH5 alpha E. coli cell line with lacZDM15 mutation as host cells, than we prepared a medium consisting of: | ||
| + | * 2.0 g/l peptone | ||
| + | * 0.1 g/l yeast extract | ||
| + | * 0.3 g/l K2HPO4 | ||
| + | * 2.1 g/l NaHCO3 | ||
| + | * a pinch of the indiciator powder: Bromothymol blue | ||
| + | * 10 g/l lactose | ||
| + | * ''Autoclaved or filter-sterilized water sample dilutions'' | ||
| + | |||
| + | We spiked these media with transformed bacterial cells containing the arsenic biosensor system, and incubated 24 hours at 37°C. | ||
| + | |||
| + | |||
| + | === Results === | ||
| + | |||
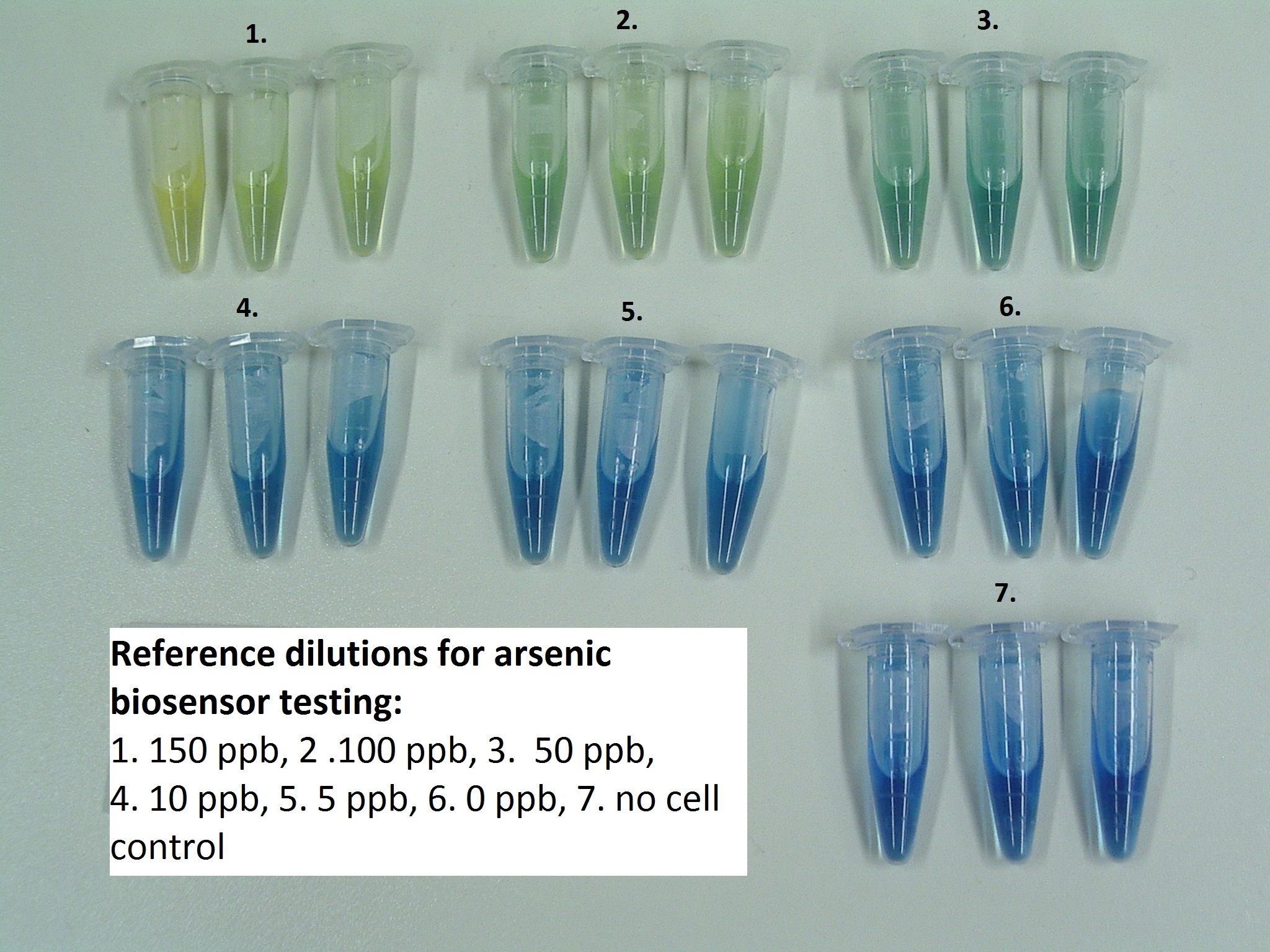
| + | We received that the part works also on real world samples, not only on samples made of water and sodium-arsenate. The colour change of Bromothymol Blue seemed to be directly proportional to the concentration of arsenic. According to the reference dilutions made from Na-arsenate and MilliQ water the concentration of arsenic in our collected samples is at least 15-fold higher than the WHO recommended limit (Fig.1., Fig.2., Fig.3.). | ||
| + | |||
| + | [[File:arzén.jpg|400px|center|thumb|Fig.1.: Arsenic reference dilutions for estimating arsenic concentration of real world samples after 24 hours of incubation.]] | ||
| + | |||
| + | [[File:arzén1.jpg|400px|center|thumb|Fig.2.: Dilutions of Szegfű street sample after 24 hours of incubation.]] | ||
| + | |||
| + | [[File:arzén2.jpg|400px|center|thumb|Fig.3.: Dilutions of Madách street sample after 24 hours of incubation.]] | ||
| + | |||
| + | As the result of the successful cooperation, an article was published on the topic: | ||
| + | |||
| + | K. de Mora, N. Joshi, B. L. Balint et al. "A pH-based biosensor for detection of arsenic in drinking water" Anal Bioanal Chem. 2011 May;400(4):1031-9. Epub 2011 Mar 27. | ||
| + | |||
| + | Link: [http://www.ncbi.nlm.nih.gov/pubmed?term=balint%20arsenic] | ||
Revision as of 21:52, 21 September 2011
Testing of arsenic biosensor
Background
Arsenic is a serious groundwater contaminant of major public health significance. During the 1970s, due to a high incidence of waterborne disease, millions of tube wells were drilled in Bangladesh to provide drinking water. Unfortunately, years later, many instances of ill health were found to be associated with consumption of this water, and it became apparent that many of the wells had inadvertently been drilled through arsenic bearing sediments and that the water was contaminated with arsenic in the form of arsenate and arsenite anions. Arsenic is a serious poison, and chronic consumption leads to arsenicosis, with symptoms such as skin lesions and cancers. The WHO limit for arsenic in drinking water is 10 ppb, though a more relaxed limit of 50 ppb is still in operation in many countries. Many wells exceed these limits. Similar contaminated groundwater has also been found to occur in many other countries, and one estimate suggests that 100 million people worldwide may be at risk. There is therefore a need for methods for testing and monitoring arsenic levels. Various highly accurate laboratory methods are available, but for large scale use in less developed regions, it would be preferable to have simple and cheap test kits which can be used in the field.
In 2006, Team Edinburgh have developed a genetically engineered machine which was able to detect arsenite/arsenate ions in water samples. They particitaped first time and won first prize in the Best Real World Application category.To see the part (BBa_33203) visit the Registry of Standard Biological Parts here: [http://partsregistry.org/Part:BBa_J33203].
As the South-East of Hungary is highly affected by the arsenic problem (there are wells in which the level of arsenic exceeds the allowed limit), we had the possibility to use real-world samples for testing. The current situation of the arseinc problem in the drinking water of the south east region of Hungary can be found here: [1]
As the Edinburgh team tested the system only on distilled water samples spiked with sodium-arsenate, we contacted the team and offered our help to test the machine on real-world samples to prove that it works not only on artificial but on natural samples as well (with many different compounds that may interfere with the part's action). We also sent them twenty sterilized samples collected by a Local Public Health Organisation from different wells - this organisation has also provided data on arsenic concentrations gained from flame atomic absorption spectrometry measurements.
Method of testing
The contact person of Team Edinburgh 2006 sent us the protocol should be used according to their experiments. We collected samples from two wells which were known to contain elevated arsenic concentration (Szegfű and Madách street, Békéscsaba, Hungary). Find these places using Google Maps: [http://maps.google.com/maps?f=q&source=s_q&hl=hu&q=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&sll=37.09024,-96.503906&sspn=34.945679,92.285156&ie=UTF8&cd=1&geocode=FeAfyAIdRVlBAQ&split=0&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ll=46.666579,21.059632&spn=0.021204,0.044546&t=h&z=15], [http://maps.google.com/maps?f=q&source=s_q&hl=hu&geocode=&q=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&sll=46.666579,21.059632&sspn=0.021204,0.044546&g=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ie=UTF8&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&ll=46.666314,21.070104&spn=0.021204,0.044546&t=h&z=15]
We used DH5 alpha E. coli cell line with lacZDM15 mutation as host cells, than we prepared a medium consisting of:
- 2.0 g/l peptone
- 0.1 g/l yeast extract
- 0.3 g/l K2HPO4
- 2.1 g/l NaHCO3
- a pinch of the indiciator powder: Bromothymol blue
- 10 g/l lactose
- Autoclaved or filter-sterilized water sample dilutions
We spiked these media with transformed bacterial cells containing the arsenic biosensor system, and incubated 24 hours at 37°C.
Results
We received that the part works also on real world samples, not only on samples made of water and sodium-arsenate. The colour change of Bromothymol Blue seemed to be directly proportional to the concentration of arsenic. According to the reference dilutions made from Na-arsenate and MilliQ water the concentration of arsenic in our collected samples is at least 15-fold higher than the WHO recommended limit (Fig.1., Fig.2., Fig.3.).
As the result of the successful cooperation, an article was published on the topic:
K. de Mora, N. Joshi, B. L. Balint et al. "A pH-based biosensor for detection of arsenic in drinking water" Anal Bioanal Chem. 2011 May;400(4):1031-9. Epub 2011 Mar 27.
Link: [http://www.ncbi.nlm.nih.gov/pubmed?term=balint%20arsenic]
 "
"