Team:Wageningen UR/Project/CompleteProject1Description
From 2011.igem.org
(→Synchroscillator) |
(→Synchroscillator) |
||
| Line 74: | Line 74: | ||
| - | Due to the tendency of interconnected positive and negative feedback loops to reach a steady state rather than sustaining oscillations, the external conditions need to be precisely controlled in order for the system to produce synchronized oscillations. In this system, the only parameters relevant to oscillatory gene expression that can be controlled during operation are the cell density and the external AHL degradation rate (see model for details). | + | Due to the tendency of interconnected positive and negative feedback loops to reach a steady state rather than sustaining oscillations, the external conditions need to be precisely controlled in order for the system to produce synchronized oscillations. In this system, the only parameters relevant to oscillatory gene expression that can be controlled during operation are the cell density and the external AHL degradation rate (see [https://2011.igem.org/Team:Wageningen_UR/Project/ModelingProj1 model] for details). |
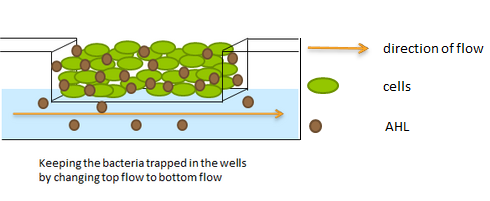
| - | However, maintaining a constant cell density over extended periods of time while independently varying the flow rate (AHL degradation) is difficult to achieve using traditional cultivation and measurement set-ups. In order to control these parameters while keeping the cells microscope-accessible, we developed a custom fluidic platform that can trap cells and allow them to remain viable over the duration of an experiment while maintaining a constant and reproducible cell density (See | + | However, maintaining a constant cell density over extended periods of time while independently varying the flow rate (AHL degradation) is difficult to achieve using traditional cultivation and measurement set-ups. In order to control these parameters while keeping the cells microscope-accessible, we developed a custom fluidic platform that can trap cells and allow them to remain viable over the duration of an experiment while maintaining a constant and reproducible cell density (See [https://2011.igem.org/Team:Wageningen_UR/Project/Devices flow chamber] page for details). |
| Line 81: | Line 81: | ||
'''Double Tunable Synchronized Oscillator''' | '''Double Tunable Synchronized Oscillator''' | ||
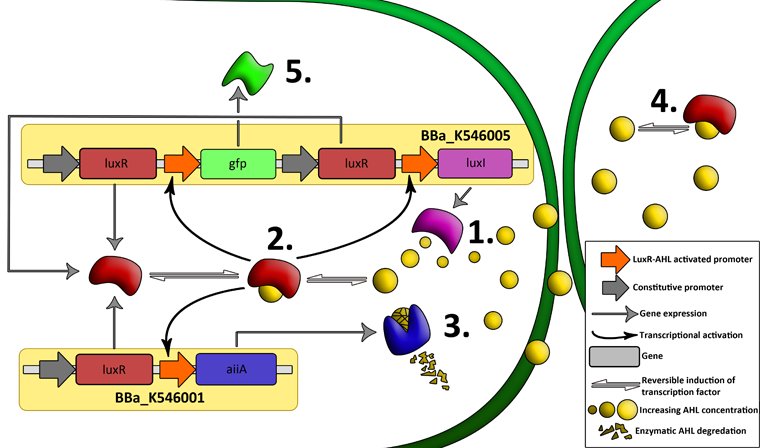
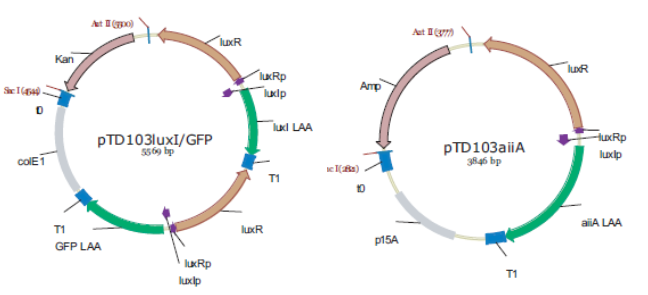
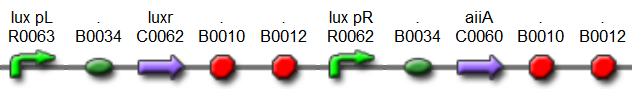
| - | The aforementioned constraints are due to the fact that there are no means by which the expression kinetics of LuxI and AiiA can be externally influenced. In order to gain more control over the expression dynamics, we refactored the system and introduced “tuning knobs” in the form of chemically inducible hybrid promoters (pR/LacI inducible by IPTG and pR/tetR inducible by anhydrotetracycline). | + | The aforementioned constraints are due to the fact that there are no means by which the expression kinetics of LuxI and AiiA can be externally influenced. In order to gain more control over the expression dynamics, we refactored the system and introduced “tuning knobs” in the form of chemically inducible hybrid promoters (pR/LacI inducible by IPTG and pR/tetR inducible by anhydrotetracycline). The addition of two independent control variables substantially expands the parameter space (cell density, flow rate) within which oscillations can occur. We also made the design more streamlined by taking advantage of the great diversity of available BioBrick quorum sensing parts in order to remove redundant elements and fit the entire system onto a single plasmid (a 30% reduction in size compared to the original design). Note: in order to take advantage of the tuning capabilities it is necessary to co-transform a plasmid which constitutively expresses LacI or TetR, or both. |
[[File:Dt.png|x67px|link=http://partsregistry.org/Part:BBa_K546546]] | [[File:Dt.png|x67px|link=http://partsregistry.org/Part:BBa_K546546]] | ||
| Line 88: | Line 88: | ||
=====4. Experimental verification===== | =====4. Experimental verification===== | ||
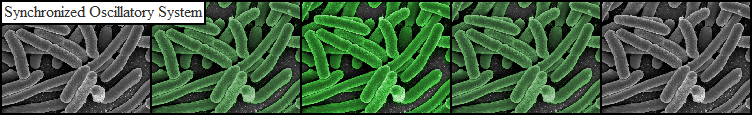
| - | The individual subcomponents of the oscillatory circuit were tested using a fluorescence photospectrometer. Changes in GFP expression relative to different AHL concentrations were measured to verify the functionality of the parts (see data page). However, due to the precise growth conditions required for synchronized oscillations to be sustained, it was necessary to adopt a more sophisticated approach to test the complete system. | + | The individual subcomponents of the oscillatory circuit were tested using a fluorescence photospectrometer. Changes in GFP expression relative to different AHL concentrations were measured to verify the functionality of the parts (see [https://2011.igem.org/Team:Wageningen_UR/Project/PartsProj1 data page]). However, due to the precise growth conditions required for synchronized oscillations to be sustained, it was necessary to adopt a more sophisticated approach to test the complete system. |
There were three requirements for the experimental set-up to test the synchronized oscillator system: | There were three requirements for the experimental set-up to test the synchronized oscillator system: | ||
| Line 96: | Line 96: | ||
b) supplying cells with fresh media to provide nutrients and remove waste (and AHL) without affecting the cell density. | b) supplying cells with fresh media to provide nutrients and remove waste (and AHL) without affecting the cell density. | ||
| - | c) directly observe cells with fluorescence microscope to perform time-lapse studies aiming at detecting changes in GFP expression over a period of hours. | + | c) directly observe cells with a fluorescence microscope to perform time-lapse studies aiming at detecting changes in GFP expression over a period of hours. |
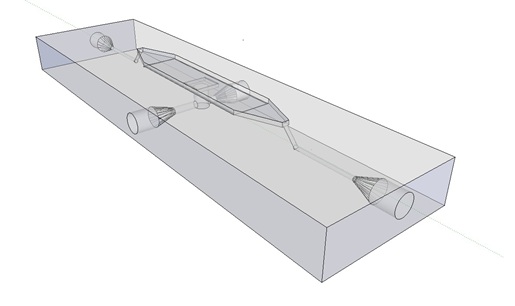
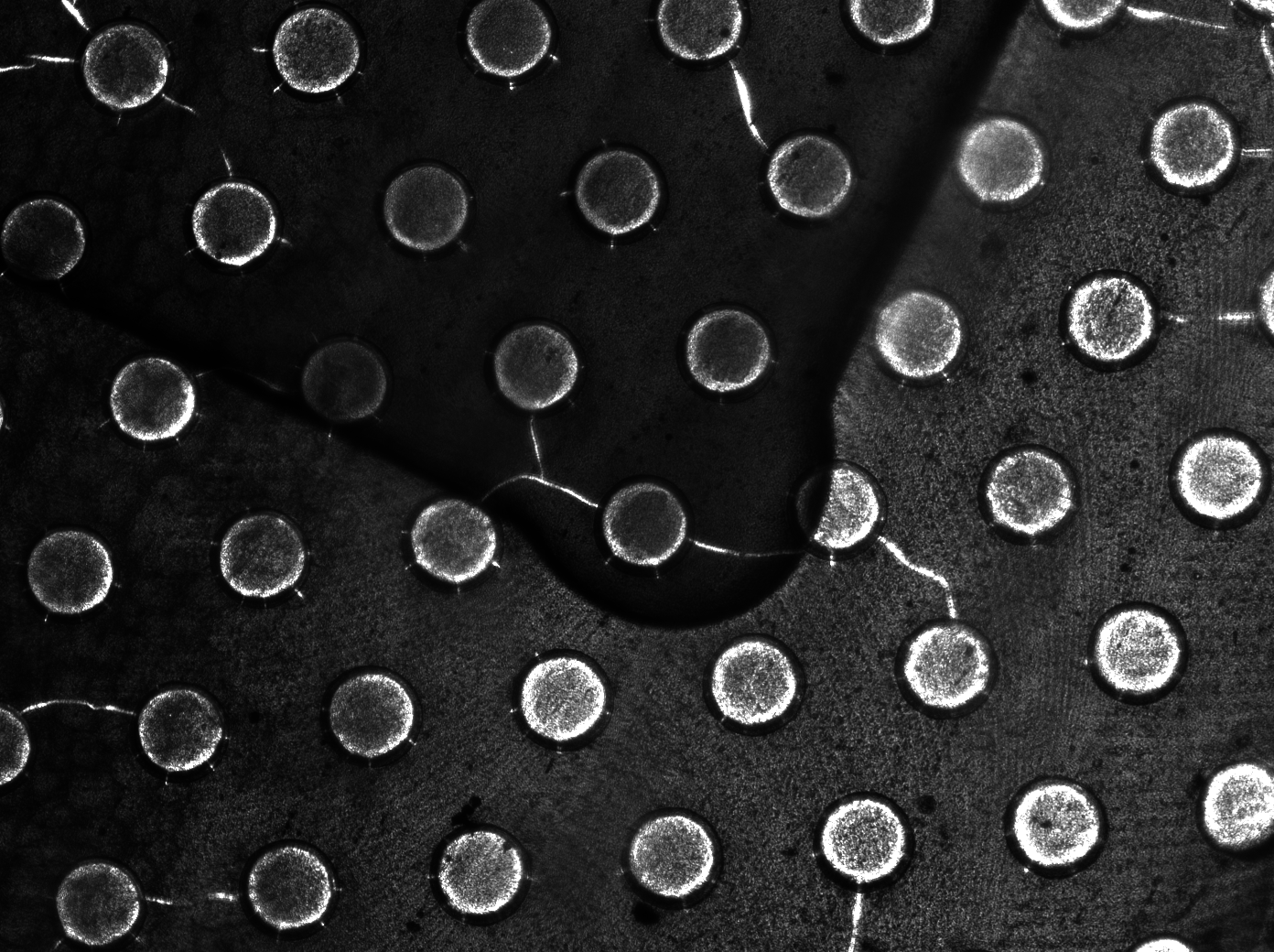
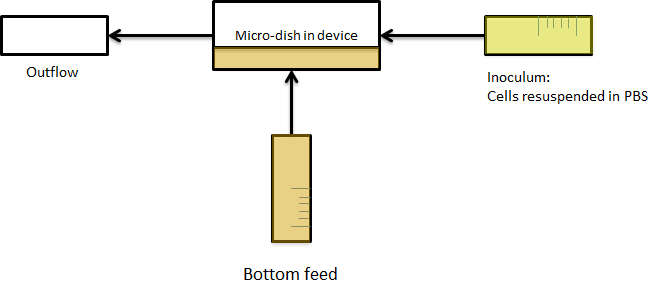
| - | Previous studies of oscillatory gene expression have employed microfluidic devices with micron-scale trapping chambers to restrict cells. However, these systems can be prohibitively expensive, require specialized accessories, and are generally not reuseable. We developed a low-cost experimental flow chamber that fulfills all of the aforementioned criteria (for detailed specifications see | + | Previous studies of oscillatory gene expression have employed microfluidic devices with micron-scale trapping chambers to restrict cells. However, these systems can be prohibitively expensive, require specialized accessories, and are generally not reuseable. We developed a low-cost experimental flow chamber that fulfills all of the aforementioned criteria (for detailed specifications see [https://2011.igem.org/Team:Wageningen_UR/Project/Devices flow chamber page]) . |
Revision as of 18:43, 21 September 2011
 "
"