Team:ETH Zurich/Biology/Detector
From 2011.igem.org
(→Acetaldehyde Sensor) |
|||
| Line 38: | Line 38: | ||
== '''Xylene Sensor''' == | == '''Xylene Sensor''' == | ||
| - | Xylene is not degraded naturally by ''E. coli''. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by included the upper Tol pathway. The activator XylR is known to work in '' | + | == Where it comes from... == |
| + | |||
| + | |||
| + | |||
| + | |||
| + | == How we used it == | ||
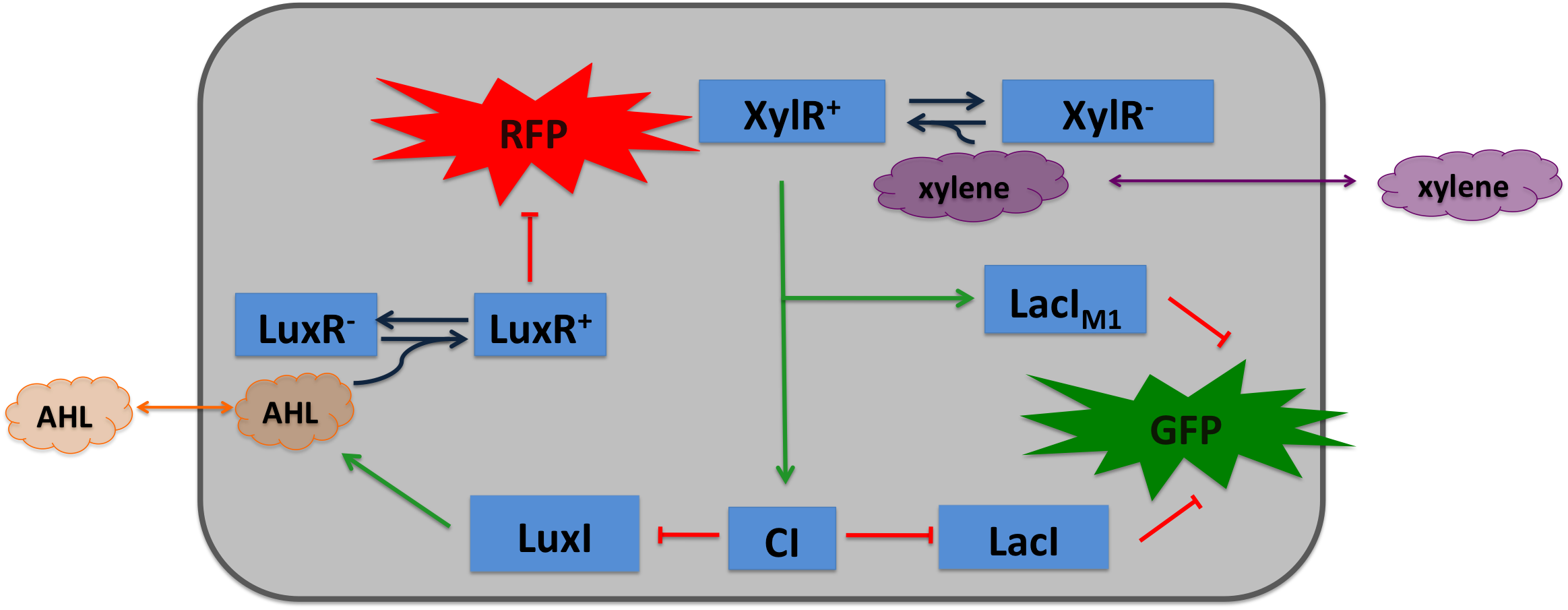
| + | Xylene is not degraded naturally by ''E. coli''. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by included the upper Tol pathway. The activator XylR is known to work in ''E.coli'' as in ''Pseudomonas putida''. In this second case we used XylR as a positive input for our bandpassfilter. | ||
Revision as of 20:39, 20 September 2011
| Sensors |
| ||
| Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology a lot of sensors for such components exist mostly to induce their degradation. One is the acetaldeyhde system in aspergillus nidulans, the activator AlcR binds to it operator site if acetaldeyhde is present [1]. An other one is the xylene sensing system in Pseudomonas putida [2] and the one for styrene in Pseudomonas sp. [3], both also work as activators. | |||
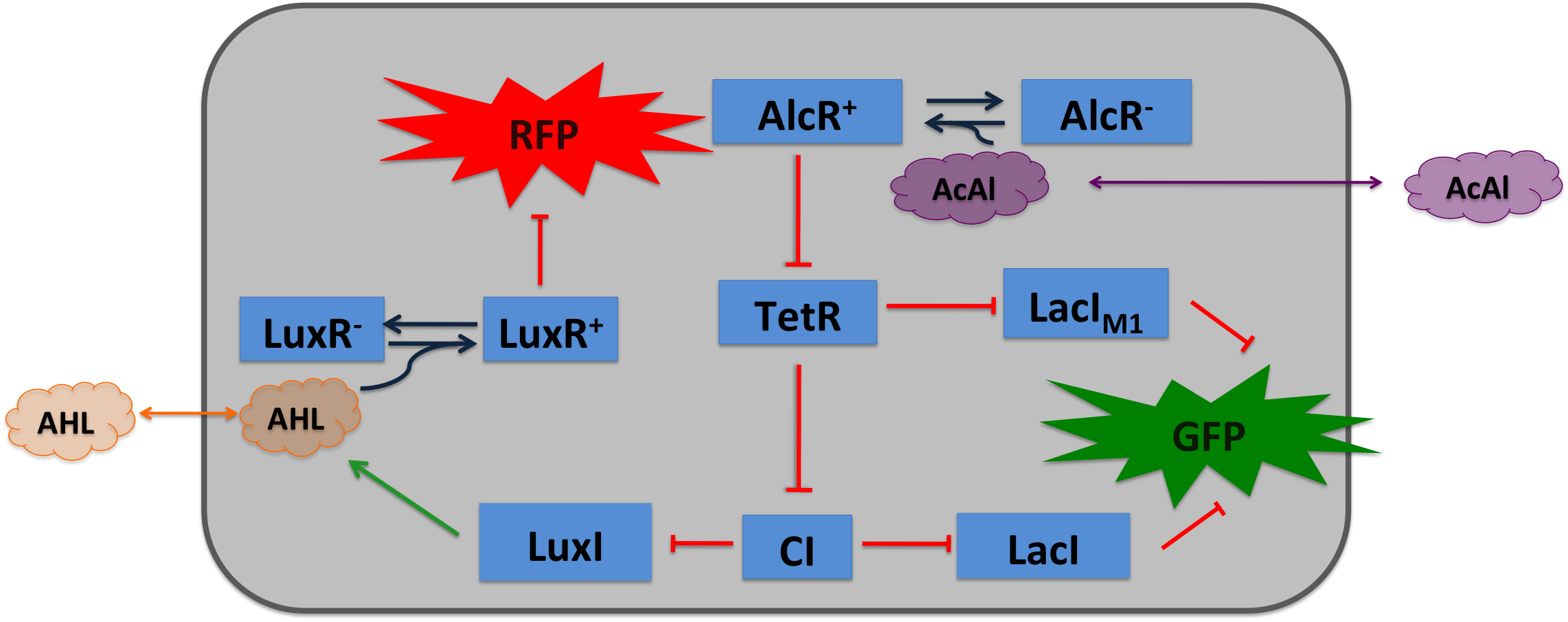
Acetaldehyde SensorWhere it comes from...In aspergillus nidulans the alcR gene encodes a regulator protein which induces the expression of the ethanol utilization (alc) pathway if the co-inducer acetaldehyde is present. Ethanol is converted to acetaldehyde by the alcohol dehydrogenase, acetaldehyde is afterward metabolized to acetate by the aldehyde dehydrogenase. Both genes alcA and aldA are regulated by AlcR. AlcR also regulate its one expression by autoactivation. The AlcR DNA binding domain contains a zinc binuclear cluster, which can bind either to symmetric and assymetric sites with same affinity. It binds as a monomer, but 2 molecules can bind to inverted repeats in a noncoperative manner. For high affinity binding additional DNA sequences upstream the zinc cluster were identified.
How we used itAcetaldehyde is naturally degraded by E. coli generating a concentration gradient in our tube. At the position with the required concentration of acetaldehyde for the bandpass-filter GFP is expressed, at all other position it is repressed, resulting in a single GFP band. By tuning the input of acetaldehyde in the tube the GFP band moves. If the acetaldehyde concentration reaches a certain very high level the whole tube expresses GFP.
|
Xylene SensorWhere it comes from...How we used itXylene is not degraded naturally by E. coli. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by included the upper Tol pathway. The activator XylR is known to work in E.coli as in Pseudomonas putida. In this second case we used XylR as a positive input for our bandpassfilter.
|
References[1] [http://www.ncbi.nlm.nih.gov/pubmed/2834622 R. Locklngton, C. Scazzocchio, D. Sequeval, M. Mathieu, B. Felenbok: Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans, Mol Microbiol., 1987, 1: 275-81] [2] [http://aem.asm.org/cgi/content/abstract/64/2/748 Sven Panke, Juan M. Sánchez-Romero, and Víctor de Lorenzo: Engineering of Quasi-Natural Pseudomonas putida Strains for Toluene Metabolism through an ortho-Cleavage Degradation Pathway, Appl Environ Microbiol, 1998, 64: 748-751] [3] [http://aem.asm.org/cgi/content/abstract/64/6/2032 Sven Panke, Bernard Witholt, Andreas Schmid, and Marcel G. Wubbolts: Towards a Biocatalyst for (S)-Styrene Oxide Production: Characterization of the Styrene Degradation Pathway of Pseudomonas sp. Strain VLB120, Appl Environ Microbiol, 1998, 64: 2032-2043] |
 "
"