Team:EPF-Lausanne/Our Project/Assembly/Plac
From 2011.igem.org
| Line 1: | Line 1: | ||
| - | {{:Team:EPF-Lausanne/Templates/ | + | {{:Team:EPF-Lausanne/Templates/ReporterHeader|title=Plac promoter characterization}} |
We used a medium-strength Plac promoter that had been designed by Henrike Niederholtmeyer and put it into biobrick format. We characterized it by coupling the promoter to RFP and adding increasing concentrations of IPTG. | We used a medium-strength Plac promoter that had been designed by Henrike Niederholtmeyer and put it into biobrick format. We characterized it by coupling the promoter to RFP and adding increasing concentrations of IPTG. | ||
Revision as of 20:27, 20 September 2011
Plac promoter characterization
Back to Reporter systems || Ptet | Plac | Plasmid detailsWe used a medium-strength Plac promoter that had been designed by Henrike Niederholtmeyer and put it into biobrick format. We characterized it by coupling the promoter to RFP and adding increasing concentrations of IPTG.
We didn't transform a LacI gene in the DH5alpha cells. Still, these cells can have a basal expression of the transcription factor. By adding IPTG to the cell's medium, we make sure to inhibit any endogenous LacI expression, in order to have the maximal RFP expression that can be driven by our Plac biobrick. The plasmid used for this characterization was J61002 Plac-RFP.
IPTG induction
The DH5alphas cells, even if the plasmid we transform doesn't contain TetR, can still have a basal expression of the transcription factor. By adding ATC, we are able to inhibit this TetR basal expression, revealing the full power of Ptet as a promoter sequence.
Experimental results:
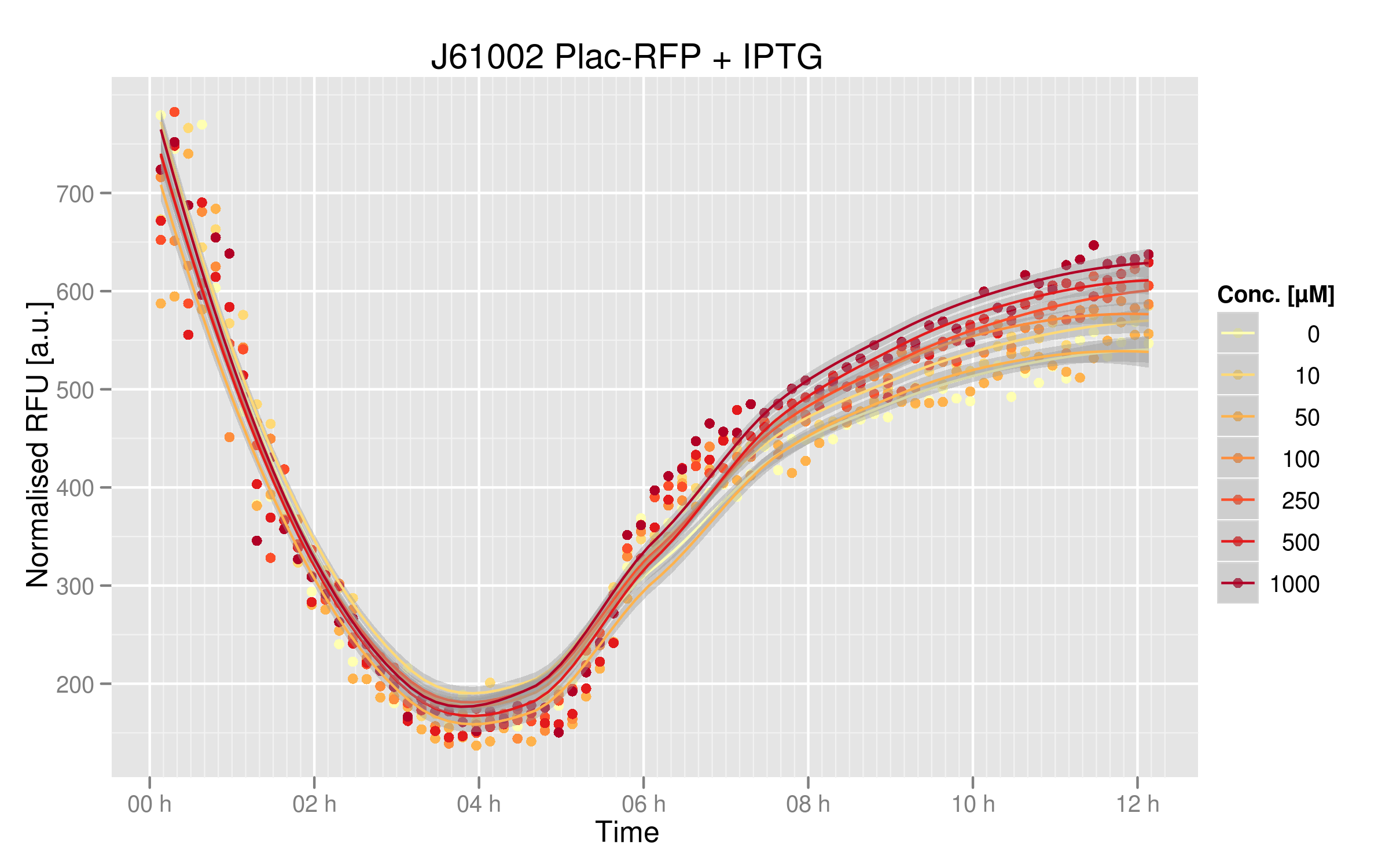
The cells treated with IPTG produced more RFP than their non-treated counterparts; this different is however not striking, showing that the basal expression of LacI by our DH5alpha cells is quite low. The normal expression of RFP driven by our Plac promoter is of 530 normalized RFUs; the highest value is 640 normalized RFUs.
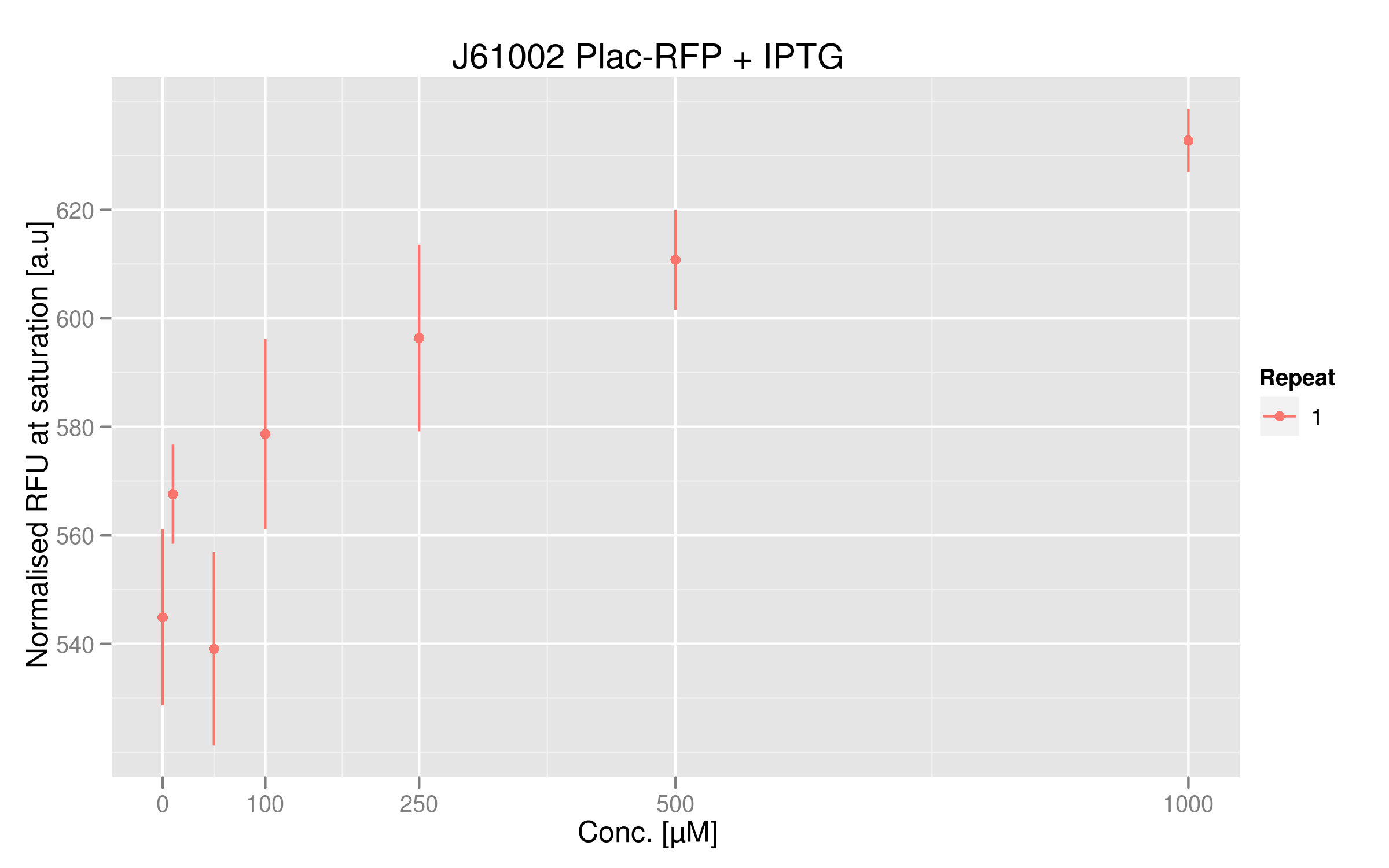
Dose-response curve
This graphs shows the action of IPTG on RFU output. Since it doesn't seem to saturate, there was perhaps still some repression by LacI going on in our cells. Interestingly, LacI does have a quantifiable action on our Ptet promoter - meaning that we can use it efficiently in our second reporter system.
 "
"