Team:ETH Zurich/Biology/Detector
From 2011.igem.org
(→Expression of AlcR in E.coli) |
|||
| Line 31: | Line 31: | ||
Given that codon usage is not the same in ''E.coli'' and ''aspergillus nidulans'', we analyzed codon usage of AlcR. | Given that codon usage is not the same in ''E.coli'' and ''aspergillus nidulans'', we analyzed codon usage of AlcR. | ||
| - | We obtained a Codon adaptation index (CAI) of 0.7 for the aspergillus nidulans protein in E.coli, especially at the beginning of the gene a few very rare codons were included. To get a fully E.coli optimized Gen, we ordered the gene codon optimized. To make some first test, we exchanged the rare codons at the beginning of the ''aspergillus nidulans'' protein with PCR. | + | We obtained a Codon adaptation index (CAI) of 0.7 for the aspergillus nidulans protein in E.coli, especially at the beginning of the gene a few very rare codons were included. To get a fully E.coli optimized Gen, we ordered the gene codon optimized. To make some first test, we exchanged the rare codons at the beginning of the ''aspergillus nidulans'' protein with PCR. With these protein we made same first Test of the expression of AlcR. |
| - | + | ''results'' | |
| + | We did a western blot to check expression of AlcR. | ||
| + | ''western blot'' | ||
|} | |} | ||
{|class="roundContainer" | {|class="roundContainer" | ||
| | | | ||
| + | |||
== '''Xylene Sensor''' == | == '''Xylene Sensor''' == | ||
Revision as of 16:39, 17 September 2011
| Sensors |
| ||
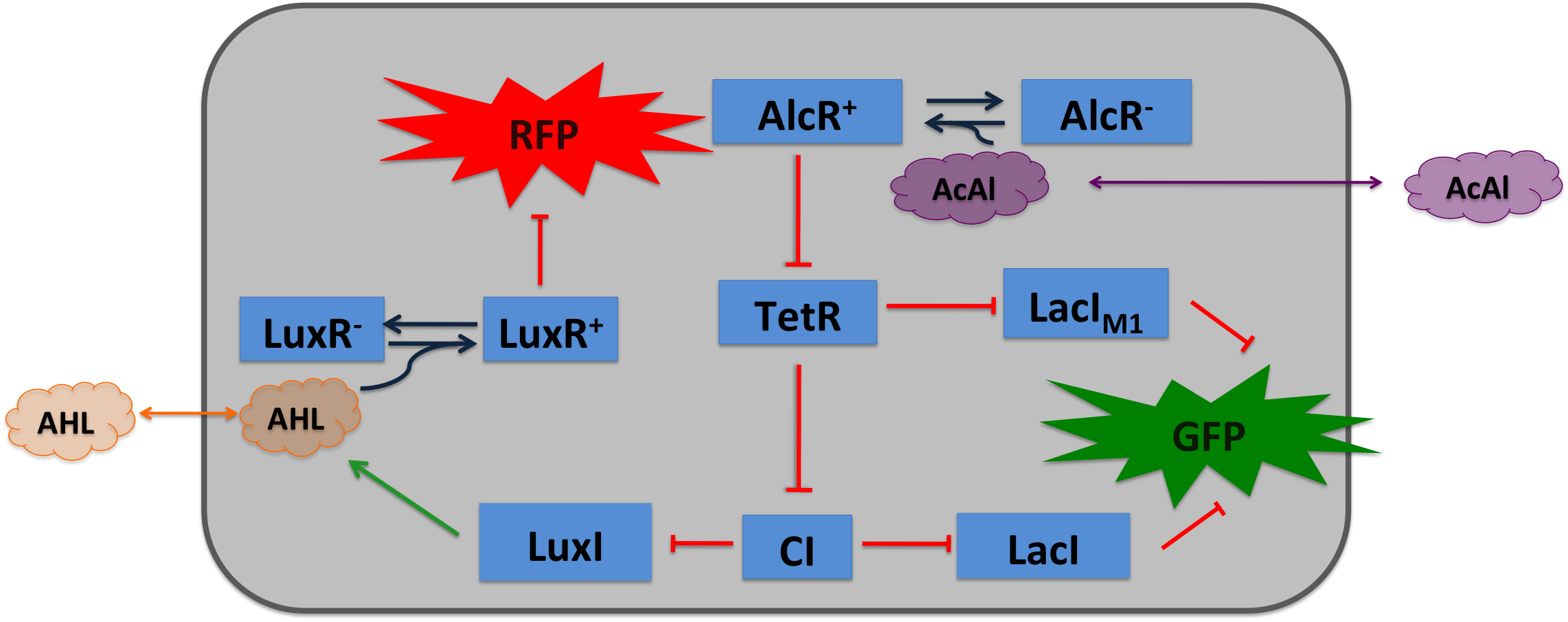
| Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology a lot of sensors for such components exist mostly to induce their degradation. One is the acetaldeyhde system in aspergillus nidulans, the activator AlcR binds to it operator site if acetaldeyhde is present [1]. An other one is the xylene sensing system in Pseudomonas putida [2] and the one for styrene in Pseudomonas sp. [3], both also work as activators. | |||
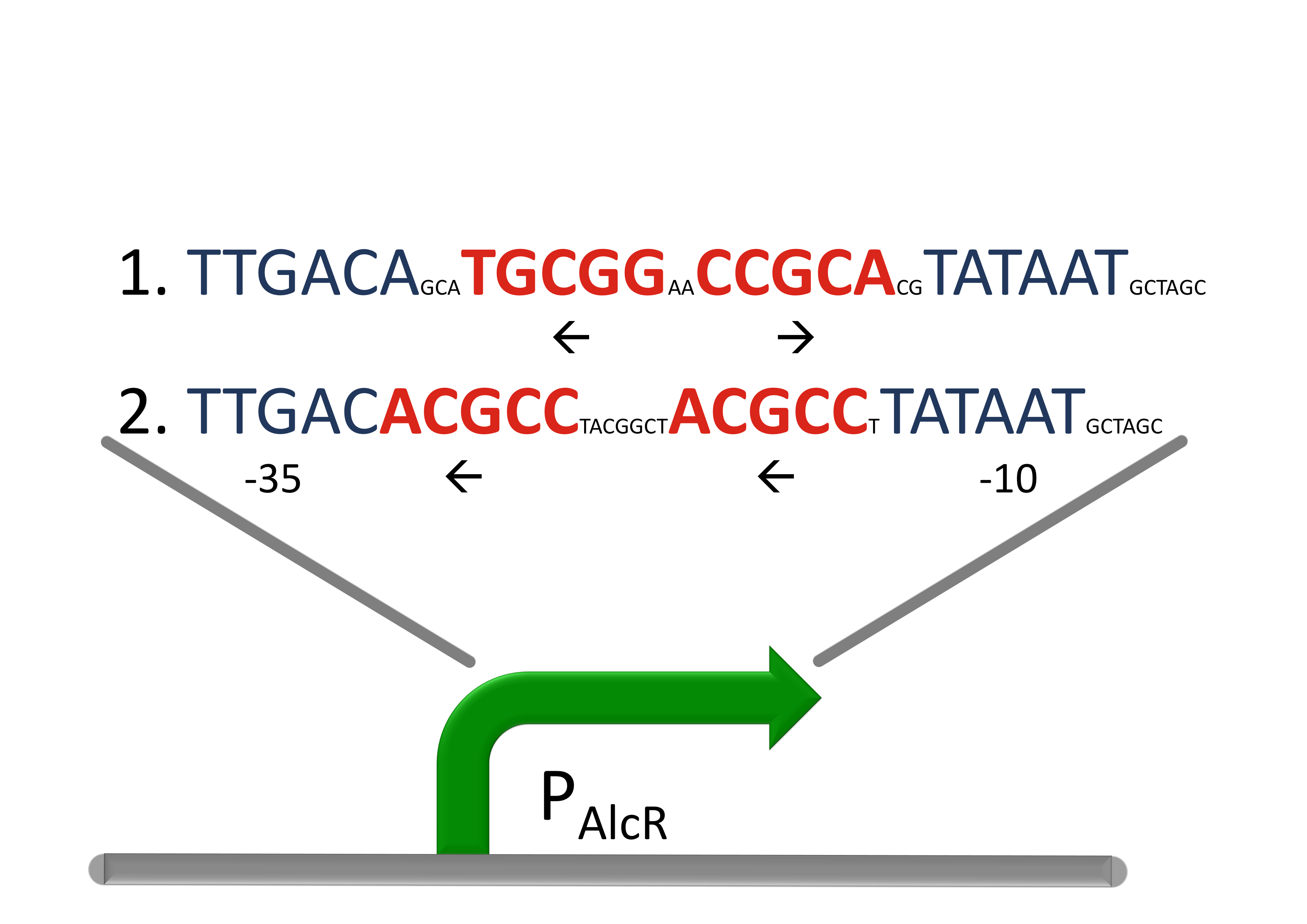
Acetaldehyde SensorAcetaldehyde is naturally degraded by e.coli generating a concentration gradient in our tube. At the position with the required concentration of acetaldehyde for the bandpass-filter GFP is expressed, at all other position it is repressed, resulting in a single GFP band. By tuning the input of acetaldehyde in the tube the GFP band moves. If the acetaldehyde concentration reaches a certain very high level the whole tube expresses GFP. Design of a AlcR/acetaldehyde repressed promoter PAlcRBecause funghi activator normally does not work in e.coli, we modified AlcR in a way that it acts as a repressor. The mechanism of how AlcR activates transcription is not completely known what makes the process of redesigning it very challenging. For the promoter PAlcR the operator site of AlcR from apergillus nidulans was placed between the -10 and -35 region of the bacterial promoter. As DNA targets we used two different versions, one with direct-repeat targets and one with inverted [4]. The idea is that in present of acetaldehyde AlcR will bind to the operon and block the binding of the RNA-polymerase. This only works if the mechanism of AlcR binding does not involves conformational changes or something similar.
Expression of AlcR in E.coliGiven that codon usage is not the same in E.coli and aspergillus nidulans, we analyzed codon usage of AlcR. We obtained a Codon adaptation index (CAI) of 0.7 for the aspergillus nidulans protein in E.coli, especially at the beginning of the gene a few very rare codons were included. To get a fully E.coli optimized Gen, we ordered the gene codon optimized. To make some first test, we exchanged the rare codons at the beginning of the aspergillus nidulans protein with PCR. With these protein we made same first Test of the expression of AlcR. results We did a western blot to check expression of AlcR. western blot |
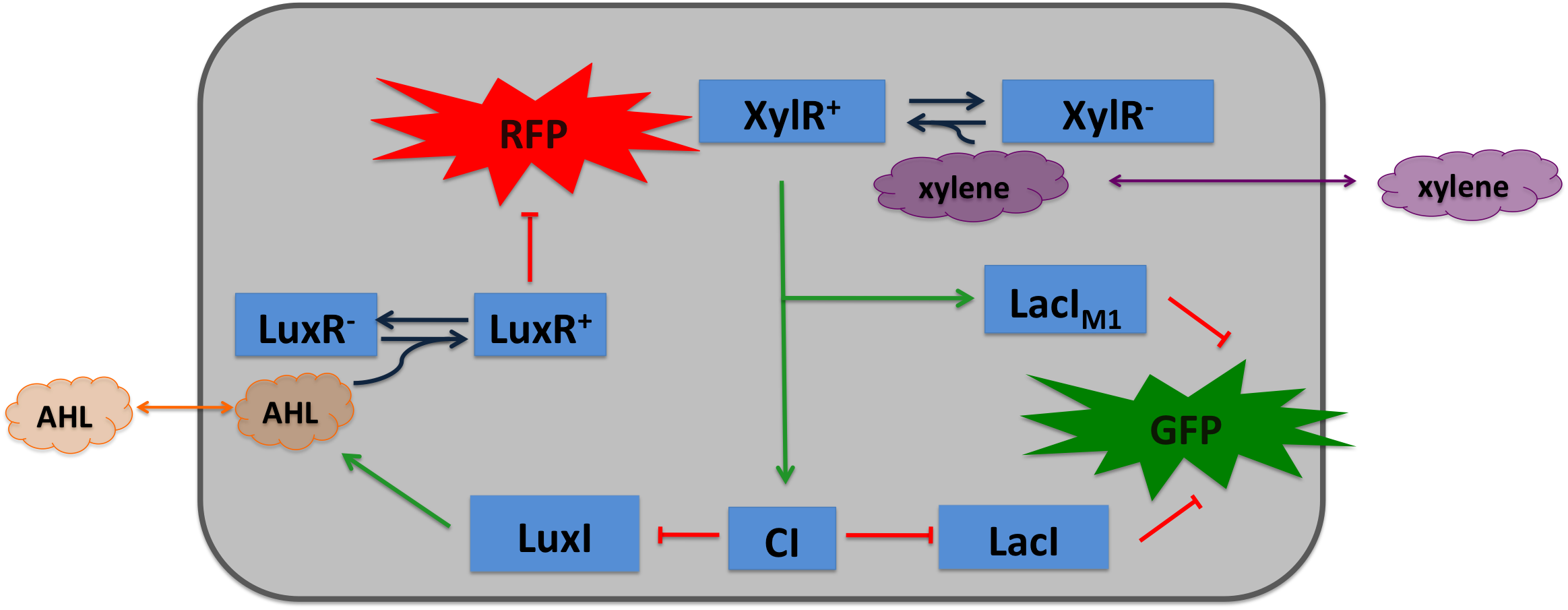
Xylene SensorXylene is not degraded naturally by e.coli. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by included the upper Tol pathway. The activator XylR is known to work in e.coli as in Pseudomonas putida
|
References[1] [http://www.ncbi.nlm.nih.gov/pubmed/2834622 R. Locklngton, C. Scazzocchio, D. Sequeval, M. Mathieu, B. Felenbok: Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans, Mol Microbiol., 1987, 1: 275-81] [2] [http://aem.asm.org/cgi/content/abstract/64/2/748 Sven Panke, Juan M. Sánchez-Romero, and Víctor de Lorenzo: Engineering of Quasi-Natural Pseudomonas putida Strains for Toluene Metabolism through an ortho-Cleavage Degradation Pathway, Appl Environ Microbiol, 1998, 64: 748-751] [3] [http://aem.asm.org/cgi/content/abstract/64/6/2032 Sven Panke, Bernard Witholt, Andreas Schmid, and Marcel G. Wubbolts: Towards a Biocatalyst for (S)-Styrene Oxide Production: Characterization of the Styrene Degradation Pathway of Pseudomonas sp. Strain VLB120, Appl Environ Microbiol, 1998, 64: 2032-2043] [4] [http://www.ncbi.nlm.nih.gov/pubmed/11550794 Beatrice Felenbok, Michel Flipphi and Igor Nikolaev: Ethanol Catabolism in Aspergillus nidulans: A Model System for Studying Gene Regulation, Progress in Nucleic Acid Research and Molecular Biology, 69: 149-204] |
 "
"