Team:Cambridge/Experiments/Reflectin Thin Films I
From 2011.igem.org
(→Reflectin Thin Films I) |
|||
| (27 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_HEAD}} | ||
| + | |||
=Reflectin Thin Films I= | =Reflectin Thin Films I= | ||
The aim of these experiments was to reproduce the flowcoating experiments reported in the literature and pioneer spincoating techniques in addition to performing controls to confirm that any properties of the films made were due to reflectin and not the solvent used in the preparation of the films. | The aim of these experiments was to reproduce the flowcoating experiments reported in the literature and pioneer spincoating techniques in addition to performing controls to confirm that any properties of the films made were due to reflectin and not the solvent used in the preparation of the films. | ||
| - | == | + | ==Method== |
| - | The | + | Below is the generic method we implemented to obtain thin films on a silicon substrate. The concentration of reflectin in HFIP used was 10% weight by weight. Being the first batch of reflectins the purity of the samples was questionable however we did observe a pink hue on resuspension in HFIP which correlates with the observations of Wendy Crookes one of the original researchers in reflectin. |
| + | *Silicon wafers were [https://2011.igem.org/Team:Cambridge/Protocols/Substrate_Preparation_for_Flow_Coating_and_Spin_Coating prepared] for use as a substrate for our thin films. | ||
| + | *Reflectin pellets obtained via our [https://2011.igem.org/Team:Cambridge/Experiments/Protein_Purification first protein purification protocol] were resuspended in HFIP before being [[Team:Cambridge/Protocols/Spin_Coating| spincoated]] or [[ Team:Cambridge/Protocols/Flow_coating | flowcoated]] onto the wafers. | ||
| + | *The thin films were produced using 10% weight by weight reflectin-HFIP solution with variable amounts of solution used to achieve a compromise between limited resources and achieving a full coating of the substrate. | ||
| + | *The manufactured thin films were analysed under an optical microscope equipped with 5x, 20x and 100x objective lenses | ||
==Results== | ==Results== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ===Observations=== | |
| - | + | <center> | |
| + | {| | ||
| + | | [[File:P8233269.JPG|200px|thumb|center|Our first reflectin thin film spun 3000 rpm for 1min, non heat treated,34 μl]] | ||
| + | | [[File:Cam Reflectin Thin Film Micrograph 1.jpg|200px|thumb|center|The same reflectin thin film under the microscope 20x objective lens]] | ||
| + | | [[File:Cam_HFIP_only_control_thinfilm.jpg | thumb | 200px | center | HFIP only control produced with a 14μl droplet. Note the black residue left as the solvent evaporated off. This residue could not be removed by ethanol, acetone or pressure wash]] | ||
| + | |}</center> | ||
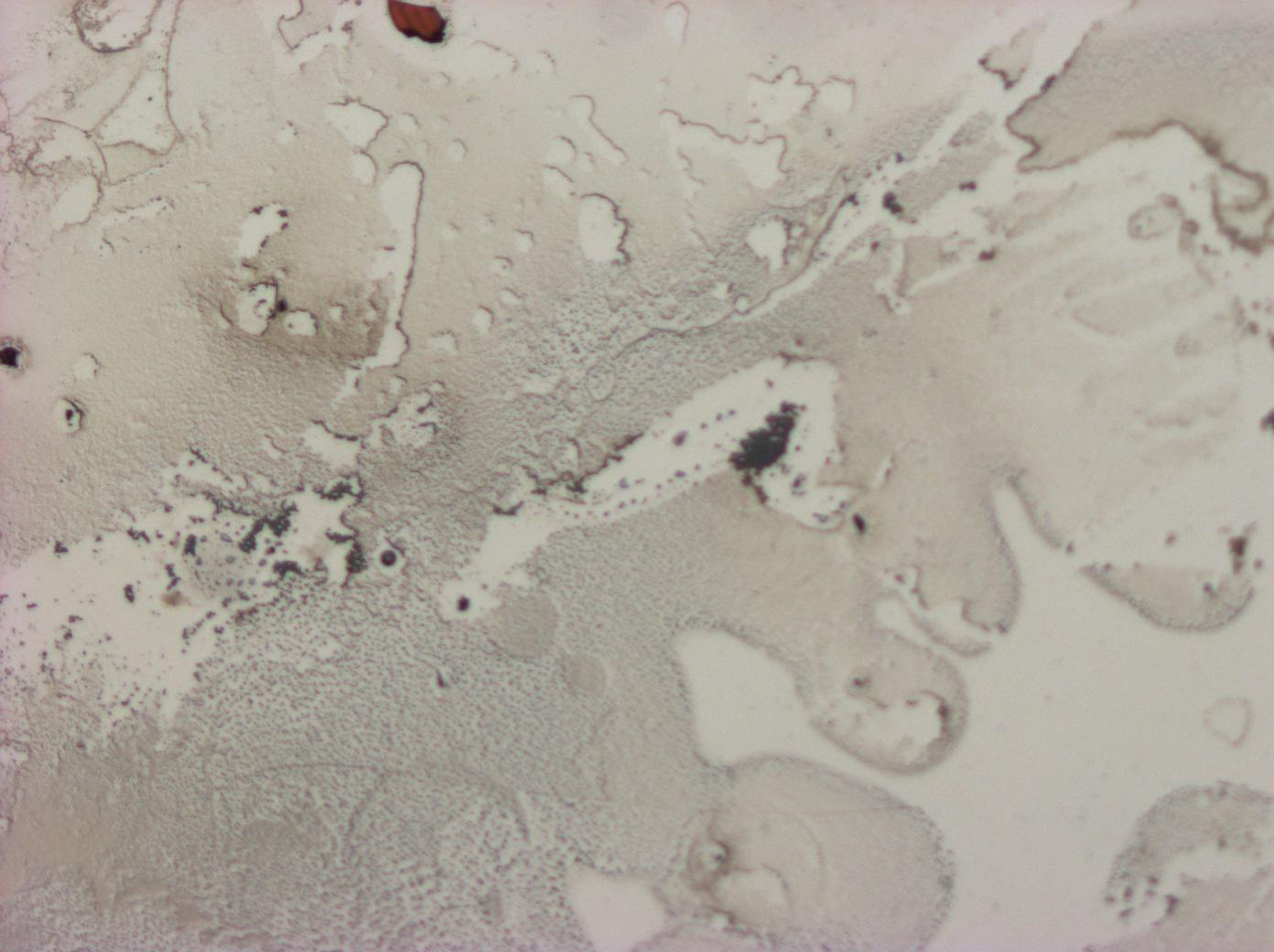
| + | *The wide spectrum of colours observed from our first reflectin thin films indicate that we had obtained a thin film of high refractive index, as we would expect if our pellets did indeed contain reflectin. The formula for the observed reflected wavelength for thin films is given by: | ||
| + | <center>λ=2 n<sub>film</sub> d cos(θ<sub>i</sub>)</center> | ||
| + | where λ=observed wavelength, n<sub>film</sub>=refractive index of film, d = thickness of film,θ<sub>i</sub>=angle of incidence between film/air(substrate) interface. | ||
| + | *Unfortunately, the colours were far from uniform. Dark objects can be seen in the centre of the regions that vary most in colour. This suggests the presence of impurities and diffuse scattering of light. The interference rings suggests local height variations around these impurities. | ||
| + | * As a control we prepared an HFIP (solvent) only thin film. This produced a predominantly colourless, dirty smear, indicating that HFIP alone could not account for the vibrancy of the thin films we obtained using reflectin pellets. The dirtiness does however suggest that the impurity of the solvent used could have been a major cause of non-uniformity | ||
| - | Safety considerations for the substrate preparation, flowcoating and spincoating techniques can be found on the relevant protocol pages. In general labcoat, safety glasses and nitrile safety gloves were worn throughout these three procedures, and all activities took place in the fume hood, except | + | ===Summary=== |
| + | * The first thin films exhibits thin film interference effects which are not due to the solvent. | ||
| + | * To further investigate the production of more uniform thin films we planned to alter the protocols we were using by namely; | ||
| + | *# Removal of insoluble impurities in reflectin solution by centrifugation | ||
| + | *# Reduced spin time from 3000rpm for 1min to 3000rpm for 30s | ||
| + | *# Use CO<sub>2</sub> pressure wash not O<sub>2</sub> plasma for finer cleaning | ||
| + | *# A further control with BSA (Bovine Serum Albumin) to test if the effects were unique to reflectin, or a feature of any proteinaceous thin film. | ||
| + | The results of these investigations are described in our [https://2011.igem.org/Team:Cambridge/Experiments/Reflectin_Thin_Films_II follow-up experiment]. | ||
| + | |||
| + | ==Safety== | ||
| + | *Safety considerations for the substrate preparation, flowcoating and spincoating techniques can be found on the relevant protocol pages. | ||
| + | *In general labcoat, safety glasses and nitrile safety gloves were worn throughout these three procedures, and all activities took place in the fume hood, except preparation of the silicon substrate. | ||
| - | {{Template:Team:Cambridge/ | + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_FOOT}} |
Latest revision as of 20:11, 21 September 2011
Contents |
Reflectin Thin Films I
The aim of these experiments was to reproduce the flowcoating experiments reported in the literature and pioneer spincoating techniques in addition to performing controls to confirm that any properties of the films made were due to reflectin and not the solvent used in the preparation of the films.
Method
Below is the generic method we implemented to obtain thin films on a silicon substrate. The concentration of reflectin in HFIP used was 10% weight by weight. Being the first batch of reflectins the purity of the samples was questionable however we did observe a pink hue on resuspension in HFIP which correlates with the observations of Wendy Crookes one of the original researchers in reflectin.
- Silicon wafers were prepared for use as a substrate for our thin films.
- Reflectin pellets obtained via our first protein purification protocol were resuspended in HFIP before being spincoated or flowcoated onto the wafers.
- The thin films were produced using 10% weight by weight reflectin-HFIP solution with variable amounts of solution used to achieve a compromise between limited resources and achieving a full coating of the substrate.
- The manufactured thin films were analysed under an optical microscope equipped with 5x, 20x and 100x objective lenses
Results
Observations
- The wide spectrum of colours observed from our first reflectin thin films indicate that we had obtained a thin film of high refractive index, as we would expect if our pellets did indeed contain reflectin. The formula for the observed reflected wavelength for thin films is given by:
where λ=observed wavelength, nfilm=refractive index of film, d = thickness of film,θi=angle of incidence between film/air(substrate) interface.
- Unfortunately, the colours were far from uniform. Dark objects can be seen in the centre of the regions that vary most in colour. This suggests the presence of impurities and diffuse scattering of light. The interference rings suggests local height variations around these impurities.
- As a control we prepared an HFIP (solvent) only thin film. This produced a predominantly colourless, dirty smear, indicating that HFIP alone could not account for the vibrancy of the thin films we obtained using reflectin pellets. The dirtiness does however suggest that the impurity of the solvent used could have been a major cause of non-uniformity
Summary
- The first thin films exhibits thin film interference effects which are not due to the solvent.
- To further investigate the production of more uniform thin films we planned to alter the protocols we were using by namely;
- Removal of insoluble impurities in reflectin solution by centrifugation
- Reduced spin time from 3000rpm for 1min to 3000rpm for 30s
- Use CO2 pressure wash not O2 plasma for finer cleaning
- A further control with BSA (Bovine Serum Albumin) to test if the effects were unique to reflectin, or a feature of any proteinaceous thin film.
The results of these investigations are described in our follow-up experiment.
Safety
- Safety considerations for the substrate preparation, flowcoating and spincoating techniques can be found on the relevant protocol pages.
- In general labcoat, safety glasses and nitrile safety gloves were worn throughout these three procedures, and all activities took place in the fume hood, except preparation of the silicon substrate.
Back to Experiments
 "
"