Team:Caltech/Week 11
From 2011.igem.org
| (4 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
Send BPA and degraded products to HPLC for analysis<br/> | Send BPA and degraded products to HPLC for analysis<br/> | ||
Send 16s for sequencing using FWD and REV. <br/> | Send 16s for sequencing using FWD and REV. <br/> | ||
| + | PCR'd R0040, K123001, B0014 and pSB3K3 for Gibson assembly of pNT002<br/> | ||
===Results=== | ===Results=== | ||
Plated 16s and WT-F87A cultures show many colonies<br/> | Plated 16s and WT-F87A cultures show many colonies<br/> | ||
| Line 15: | Line 16: | ||
* 16 LB-chlor plates | * 16 LB-chlor plates | ||
* 13 LB plates | * 13 LB plates | ||
| + | PCR'd R0040 and pSB3K3 for Gibson assembly of pNT002<br/> | ||
| + | PCR'd the DDT gene with new primers to add stop codons and biobrick ends.<br/> | ||
| + | PCR'd the DDT gene with BamHI and XbaI sites and a C-terminus his tag for insertion into pET11-a. This will allow us to overexpress the protein for purification and characterization.<br/> | ||
| + | PCR'd WT-F87A with biobrick ends.<br/> | ||
| + | Dpn 1 digested the DDT and p450 PCRs overnight<br/> | ||
| + | ==August 24== | ||
| + | Visited the San Jose Creek Water Reclamation Plant<br/> | ||
| + | Made more Gibson mix according to Joe's protocol.<br/> | ||
| + | Did double restriction digest of DDT gene and p450 PCRs with biobrick ends and pSB1C3 using EcoRI and PstI.<br/> | ||
| + | Did double restriction digest of DDT gene PCR with pET11-a insertion endings and pET11-a using BamHI and XbaI<br/> | ||
| + | Did CIP digest of pET11a and pSB1C3<br/> | ||
| + | Ligated DDT gene with biobrick ends into pSB1C3<br/> | ||
| + | Ligated p450 with biobrick ends into pSB1C3<br/> | ||
| + | Ligated DDT gene into pET11-a<br/> | ||
| + | Transformed all ligations into 50 ul aliquots of DH5a electrocompetent cells<br/> | ||
| + | Transformed 1 ul of 10 pg/ul pUC19 into 1 aliquot of DH5a electrocompetent cells<br/> | ||
| + | Started overnight of pSB1C3 so there is more DNA available to use as backbones for biobrick parts to send to the registry.<br/> | ||
| + | X-gal plating shows blue colonies; overnights of J23119-MG1655 and R0010-MG1655 to make glycerol stocks<br/> | ||
| + | ==August 25== | ||
| + | pSB1C3 concentration 118.0 ng/ul<br/> | ||
| + | Calculated transformation efficiency of DH5a electrocompetent cells is about 2x10^8 cfu/ug<br/> | ||
| + | ===Results=== | ||
| + | <table border="1"> | ||
| + | <tr> | ||
| + | <th>Name</th> | ||
| + | <th>Number of colonies</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>p450 biobricked in pSB1C3</td> | ||
| + | <td>~3400</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DDT gene biobrick now with stop codons in pSB1C3</td> | ||
| + | <td>~6400</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB1C3 ligation negative control</td> | ||
| + | <td>0</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DDT gene inserted into pET11-a</td> | ||
| + | <td>~6400</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pET11-a ligation negative control</td> | ||
| + | <td>27</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pUC 19 in DH5a electrocompetent cells</td> | ||
| + | <td>976</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>DDT gene PIPE</td> | ||
| + | <td>0</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>VPIPE negative control</td> | ||
| + | <td>0</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 + in commercial XL-10 gold</td> | ||
| + | <td>54</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 - in commercial XL-10 gold</td> | ||
| + | <td>28</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <gallery> | ||
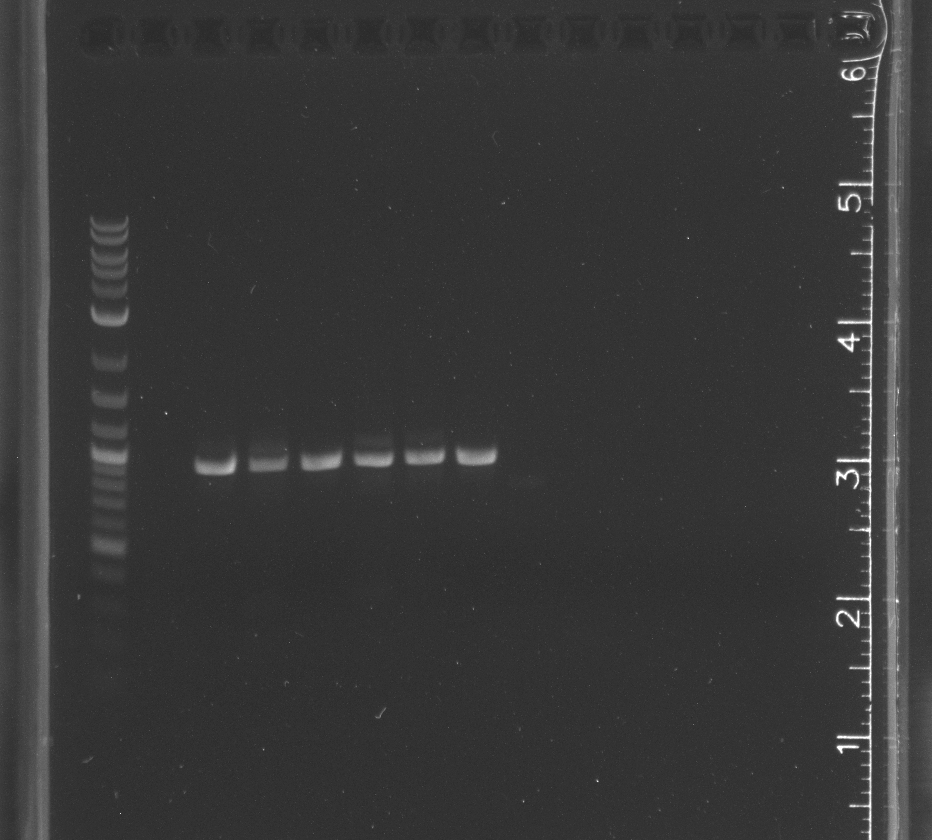
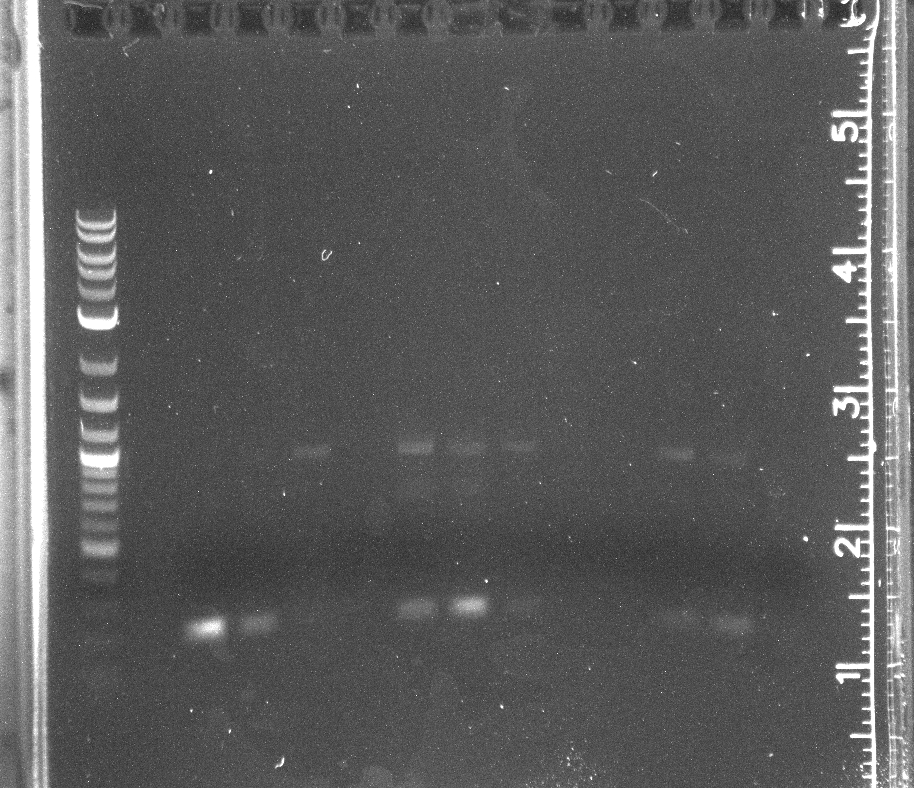
| + | File: 8-25 ddt cpcr.jpg|CPCR of DDT gene lane 1 NEB 2-log ladder, 3-8 with in biocrick form, 9-14 in pET11a | ||
| + | File: 8-25 p450 cpcr.jpg|CPCR of WT-F78A in biobrick form, lane 1 NEB 2-log ladder, 3-8 WT-F87A | ||
| + | File: 8-25 pnt002 cpcr.jpg|CPCR of pNT002 constructed by Gibson, lane 1 NEB-2 log ladder, 3-14 pNT002 | ||
| + | </gallery> | ||
| + | ==August 26== | ||
| + | Sent improved DDT biobrick and DDT in pET11a off fro sequencing<br/> | ||
| + | Added X-gal to J23119 overnights to test for expression | ||
| + | ===Results=== | ||
| + | J23119-MG1655 culture turned X-gal blue; use spectrophotometer for better measurement | ||
| + | Started culture of J23119-MG1655 in flask with glass beads for biofilm/X-gal experiment | ||
| + | ==August 27== | ||
| + | Moved from Braun 16 to Keck 040<br/> | ||
}} | }} | ||
Latest revision as of 18:53, 1 September 2011
|
Project |
August 22Send BPA and degraded products to HPLC for analysis ResultsPlated 16s and WT-F87A cultures show many colonies August 23Make experimental MG-1655 strain electrocompetent
PCR'd R0040 and pSB3K3 for Gibson assembly of pNT002 August 24Visited the San Jose Creek Water Reclamation Plant August 25pSB1C3 concentration 118.0 ng/ul Results
August 26Sent improved DDT biobrick and DDT in pET11a off fro sequencing ResultsJ23119-MG1655 culture turned X-gal blue; use spectrophotometer for better measurement Started culture of J23119-MG1655 in flask with glass beads for biofilm/X-gal experiment August 27Moved from Braun 16 to Keck 040
|
 "
"