Team:Calgary/Project
From 2011.igem.org
Emily Hicks (Talk | contribs) |
|||
| (56 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{ | + | {{Team:Calgary/Main_Header|project}} |
| + | {{Team:Calgary/ProjectBar| | ||
| + | TITLE=A Biosensor for Naphthenic Acids| | ||
| + | BODY=<html> | ||
| + | <h2>Naphthenic Acids in the Oil Sands</h2> | ||
| - | <html> | + | </html>[[Image:UofC2011_Whitbey.png|thumb|600px|center|<b>Figure 1.</b> Structure of Naphthenic Acids as discussed in Whitby, C. <i>et al.</i> 2010.]]<html> |
| - | < | + | |
| - | + | <p>Naphthenic Acids (NAs) are a group of recalcitrant and hydrophobic compounds comprising a variety of structures. All NAs contain a conserved carboxylic acid group followed by a hydrocarbon chain. Attached to this hydrophobic chain there may be between one and four hydrogenated ring systems. NA classification criteria are continually debated in the scientific community. With such diversity within this chemical class, the biodegradation of NAs is difficult to define due to the likelihood of biodegradation pathways interacting with very specific topological structures. Thus one pathway likely does not degrade all NAs, and NA research has yet to define any NA degrading pathways (Whitby, 2010).</p> | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | </html>[[Image:UofC_TailingPond.png|thumb|600px|center|<b>Figure 2.</b> Courtesy of Pembina, a picture of a tailings pond]]<html> | |
| - | + | <h2>Bitumen Extraction Process</h2> | |
| - | + | <p>NAs naturally occur in all petroleum deposits and their surfactant quality is actually useful for higher efficiency of oil sands recovery in the hot water extraction process. Post processing, the majority of NAs from the tar sands separate into the waste water aqueous fraction and are then collected in large tailings ponds along with a variety of other toxic by-products of bitumen extraction. The NAs continuously accumulate in these large tailings ponds and are allowed to settle to the bottom of these pools.</p> | |
| - | + | ||
| - | + | </html>[[Image:UofC_Extraction.png|thumb|600px|center|<b>Figure 3.</b>The extraction process from bitumen to oil, showing where waste like naphthenic acids is extracted]]<html> | |
| - | + | ||
| - | + | ||
| - | + | <h2>The Toxicity of Naphthenic Acids</h2> | |
| - | + | ||
| - | + | ||
| - | + | <p>One of the major industry concerns with NAs is their contribution to corrosion of equipment and pipelines. In addition, their surfactant nature also makes them toxic in mammalian systems allowing them to pass through and potentially disrupt cell membranes. NAs with a higher molecular weight are typically less toxic than those with a lower molecular weight, however, the lower molecular weight compounds still have significant toxicity with LC<sub>50</sub> values of less than 50 mg/L in freshwater systems. The higher the concentration of NAs in the environment the greater the potential harm to the local ecosystem (Dokhoyan and Magomedov, 1984).</p> | |
| - | + | <br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </html>[[Image:UCalgary2011_LC50_values_for_fish_and_stuff.png|thumb|600px|center|<b>Figure 4.</b> LC<sub>50</sub> values for Adult kutum, sturgeon, and roach when exposed to Napthenic Acids. Adapted from Dokholyan VK., Magomedov AK. (1983). ]]<html> | |
| - | + | <br></br> | |
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | </ | + | <h2>Monitoring Naphthenic Acids</h2> |
| - | < | + | <p>Having the ability to monitor the levels of NAs is mandated by Canadian law and would be useful in assessing whether or not any future detoxification or remediation efforts are effective. Other applications include examining the surrounding areas for seepage of NAs into ground water, and determining the extent of damage caused by oil based chemical spills. Currently, the main way to test for the presence of NAs is through gas chromatography-mass spectrometry. This method is costly, requires the experience of a trained technician, takes up to several hours per sample, and the samples must be shipped to a facility with the proper equipment for analysis. The University of Calgary’s iGEM team is working on developing a novel electrochemical biosensor which would allow for cheap, quick, and convenient on site monitoring of NAs. The bacteria used to sense the naphthenic acids would be contained inside a device, and respond specifically to these compounds. Their response would signal a change in electrochemical potential in the solution that could be used as a read-out for our device in a quantitative manner.</p> |
| - | + | </html>[[Image:TeamCalgary2011 The Vision Image 2.png|thumb|600px|center|<b>Figure 5.</b> The Vision of our project. The University of Calgary team imagined a device which could allow for on-site testing of water samples for naphthenic acids. Solutions would be placed into the device, where bacteria would recognize the concentration of naphthenic acids in solution and generate a response. This can be detected by the device and a quantitative interpretation of the data would be viewable by the user.]]<html> | |
| - | + | ||
| - | + | <h2>Engineering the Biosensor</h2> | |
| - | + | ||
| - | + | ||
| - | + | <p>Engineering the biosensor involved three main components: finding an appropriate sensory element, characterizing a novel reporter and selecting and designing tools for an appropriate chassis for our system. Information on the sensory element can be found on our <a href="https://2011.igem.org/Team:Calgary/Project/Promoter">Promoter Project</a> page. More details on our reporter can be found on the <a href="https://2011.igem.org/Team:Calgary/Project/Reporter">Reporter Project</a> page. More details on our chassis can be found on our <a href="https://2011.igem.org/Team:Calgary/Project/Chassis">Chassis Project</a> page.</p> | |
| - | + | <style> | |
| - | </ | + | #titlebar{margin-top: -6px;} |
| + | #pagetitle{padding-top: 17px;} | ||
| + | #bodycontainer{padding-top: 6px;} | ||
| + | </style> | ||
</html> | </html> | ||
| + | }} | ||
Latest revision as of 04:31, 29 September 2011









A Biosensor for Naphthenic Acids

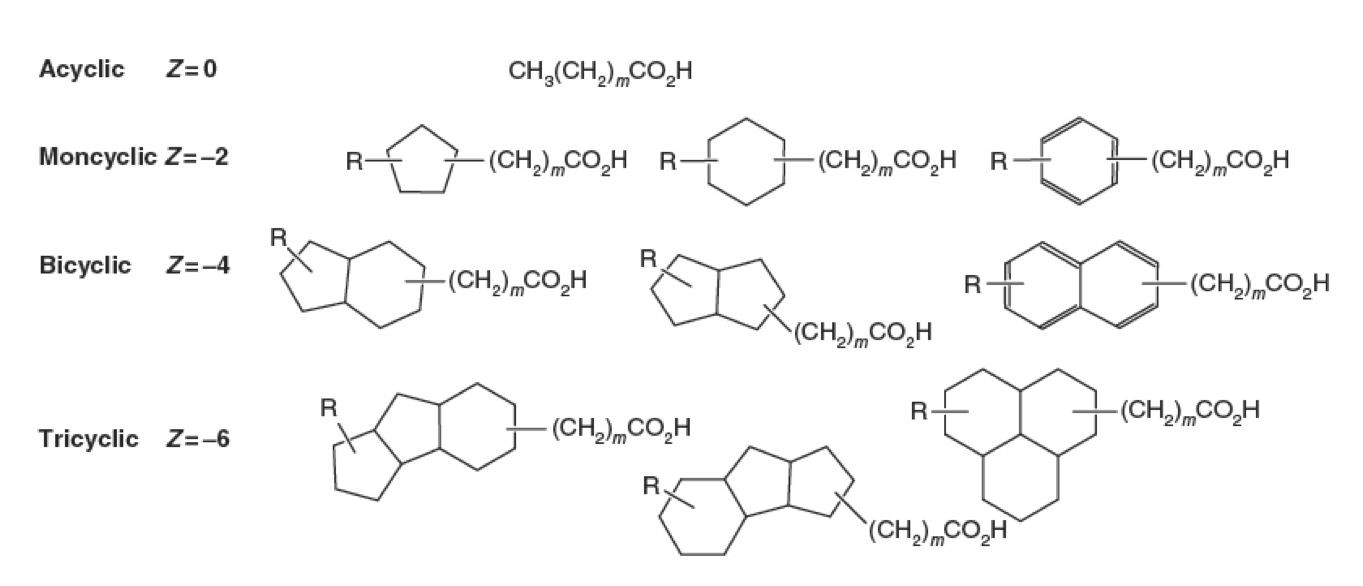
Naphthenic Acids in the Oil Sands
Naphthenic Acids (NAs) are a group of recalcitrant and hydrophobic compounds comprising a variety of structures. All NAs contain a conserved carboxylic acid group followed by a hydrocarbon chain. Attached to this hydrophobic chain there may be between one and four hydrogenated ring systems. NA classification criteria are continually debated in the scientific community. With such diversity within this chemical class, the biodegradation of NAs is difficult to define due to the likelihood of biodegradation pathways interacting with very specific topological structures. Thus one pathway likely does not degrade all NAs, and NA research has yet to define any NA degrading pathways (Whitby, 2010).
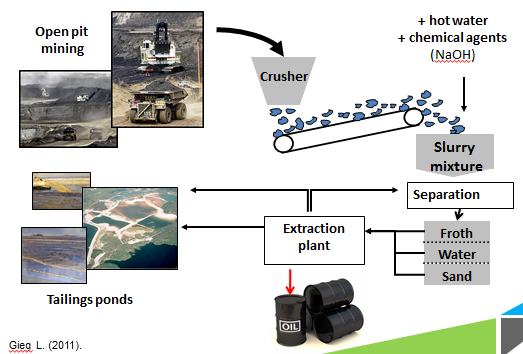
Bitumen Extraction Process
NAs naturally occur in all petroleum deposits and their surfactant quality is actually useful for higher efficiency of oil sands recovery in the hot water extraction process. Post processing, the majority of NAs from the tar sands separate into the waste water aqueous fraction and are then collected in large tailings ponds along with a variety of other toxic by-products of bitumen extraction. The NAs continuously accumulate in these large tailings ponds and are allowed to settle to the bottom of these pools.
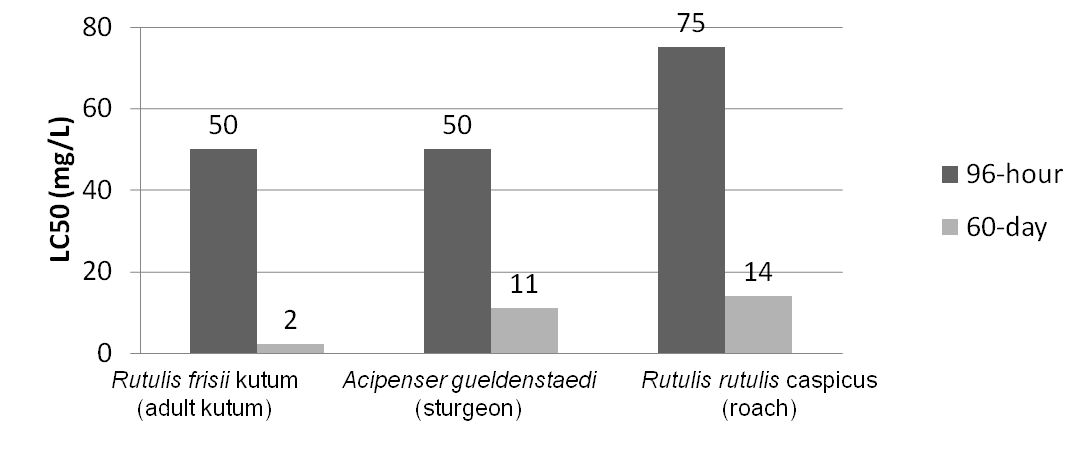
The Toxicity of Naphthenic Acids
One of the major industry concerns with NAs is their contribution to corrosion of equipment and pipelines. In addition, their surfactant nature also makes them toxic in mammalian systems allowing them to pass through and potentially disrupt cell membranes. NAs with a higher molecular weight are typically less toxic than those with a lower molecular weight, however, the lower molecular weight compounds still have significant toxicity with LC50 values of less than 50 mg/L in freshwater systems. The higher the concentration of NAs in the environment the greater the potential harm to the local ecosystem (Dokhoyan and Magomedov, 1984).
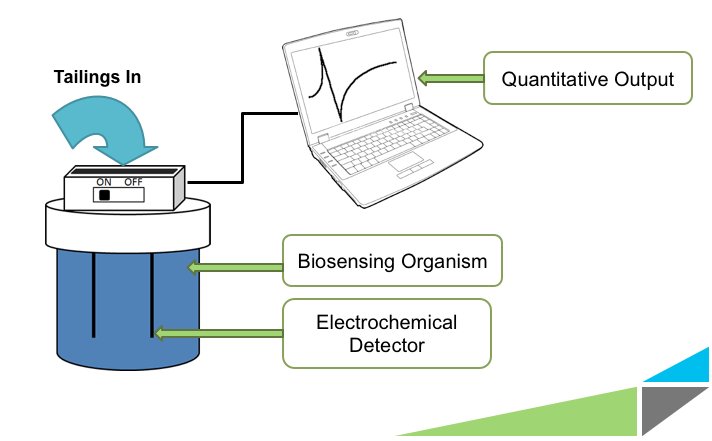
Monitoring Naphthenic Acids
Having the ability to monitor the levels of NAs is mandated by Canadian law and would be useful in assessing whether or not any future detoxification or remediation efforts are effective. Other applications include examining the surrounding areas for seepage of NAs into ground water, and determining the extent of damage caused by oil based chemical spills. Currently, the main way to test for the presence of NAs is through gas chromatography-mass spectrometry. This method is costly, requires the experience of a trained technician, takes up to several hours per sample, and the samples must be shipped to a facility with the proper equipment for analysis. The University of Calgary’s iGEM team is working on developing a novel electrochemical biosensor which would allow for cheap, quick, and convenient on site monitoring of NAs. The bacteria used to sense the naphthenic acids would be contained inside a device, and respond specifically to these compounds. Their response would signal a change in electrochemical potential in the solution that could be used as a read-out for our device in a quantitative manner.
Engineering the Biosensor
Engineering the biosensor involved three main components: finding an appropriate sensory element, characterizing a novel reporter and selecting and designing tools for an appropriate chassis for our system. Information on the sensory element can be found on our Promoter Project page. More details on our reporter can be found on the Reporter Project page. More details on our chassis can be found on our Chassis Project page.

 "
"