Team:Freiburg/Notebook/15 August
From 2011.igem.org
(Difference between revisions)
(Created page with "{{:Team:Freiburg/Templates/header}} ==<span style="color:green;">green light receptor</span>== ===NAME OF YOUR EXPERIMENT=== '''Investigators:NAME''' ==<span style="color:b...") |
|||
| (25 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Freiburg/Templates/header}} | {{:Team:Freiburg/Templates/header}} | ||
| + | <html> | ||
| + | <div id="notebook-page-header"> | ||
| + | <div id="notebook-back" width="100px" > | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook/14_August">Previous entry</a> | ||
| + | </div> | ||
| + | <div id="notebook-title"> | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook"> 15 August </a> | ||
| + | </div> | ||
| + | <div id="notebook-next"> | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook/16_August">Next entry</a> | ||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
==<span style="color:green;">green light receptor</span>== | ==<span style="color:green;">green light receptor</span>== | ||
| - | === | + | ===Order primers=== |
| - | + | ||
| - | + | ||
| + | '''Investigators: Jakob''' | ||
| + | *Order new primers for the quickchange of CcaS and CcaR | ||
| + | *I got the sequence from the iGEM-Team Uppsala THX again! | ||
==<span style="color:blue;">blue light receptor</span>== | ==<span style="color:blue;">blue light receptor</span>== | ||
| - | === | + | ===Transformation=== |
| - | '''Investigators: | + | '''Investigators: Sophie''' |
| + | Transformation of ligated parts: | ||
| + | *LovTAP | ||
| + | *Not-Gate | ||
| + | *T3 vector | ||
| + | stored in incubator at 37°C. | ||
| + | <br/> | ||
| + | <br/> | ||
==<span style="color:red;">red light receptor</span>== | ==<span style="color:red;">red light receptor</span>== | ||
| - | === | + | ===PCR for Cph8=== |
| - | '''Investigators: | + | '''Investigators:Julia''' |
| + | <br/> | ||
| + | after protocol from Uppsala. | ||
| + | PCR-Mixture for one Reaction: | ||
| + | For a 50 µl reaction use | ||
| + | |||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 32,5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| H<sub>2</sub>0 | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 10µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 5x Phusion Buffer | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer fw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer dw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| dNTPs | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| DNA-Template | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 0.5 µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Phusion (add in the end) | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |} | ||
| + | |||
| + | Primer used: | ||
| + | <br/> | ||
| + | cph8 Prefix: TTCGAATTCGCGGCCGCTTCTAGATGGCCACCACCGTACAA<br/> | ||
| + | cph8 Suffix: CCGCTACTAGTATTATTACCCTTCTTTTGTCATGCCCT<br/> | ||
| + | <br/> | ||
| + | PCR program: <br/> Temp. Time,Nr of cycles <br/> | ||
| + | 98° 5' 1x <br/> | ||
| + | 98° 30'' 10x <br/> | ||
| + | 65° 30'' <br/> | ||
| + | 72° 1'15'' <br/> | ||
| + | 98° 30'' 20x <br/> | ||
| + | 72° 1'15'' <br/> | ||
| + | 72° 7' 1x <br/> | ||
| + | 4° ∞ <br/> | ||
| + | <br/> | ||
| + | |||
| + | Template: Voights plasmid (10x dilution)<br/> | ||
| + | Amplicon: 2235 bp<br/> | ||
| + | |||
| + | Elongation time Phusion 67,05 sec | ||
==<span style="color:orange;">Lysis cassette</span>== | ==<span style="color:orange;">Lysis cassette</span>== | ||
| - | === | + | ===2A Assembly of the Lysis Cassette (S4+S15)=== |
| - | '''Investigators: | + | '''Investigators:Theo''' |
| + | Since 3A assemly doesn't want to help us, we are going to try a 2A Assembly of S4 with S15.<br> | ||
| + | S4 is cut with SpeI and PstI.<br> | ||
| + | S15 is cut with XbaI and PstI, and is to be run on a gel, cut, isolated, ligated with S4 and finally transformed in competent cells.<br> | ||
| + | |||
| + | After the gel with S15 was run, I noticed there was no band to be seen and our instructor told me it was because I had not used a lot of DNA (500ng). | ||
| + | <br> | ||
| + | |||
| + | I am going to repeat this tomorrow. | ||
==<span style="color:grey;">Precipitator</span>== | ==<span style="color:grey;">Precipitator</span>== | ||
| - | === | + | ===Ligation=== |
| + | |||
| + | '''Investigators: Sophie'''<br/> | ||
| + | continue from experiment: Cloning (12.8.11)<br/> | ||
| + | Project name: inducible promoter for pbd<br/> | ||
| + | Vector: psb1T3<br/> | ||
| + | Inserts: IPTG-Promoter,Pbd-GFP<br/> | ||
| + | samples stored in Ruediger's box<br/> | ||
| + | |||
| + | ===Transformation=== | ||
| + | |||
| + | '''Investigators: Sophie'''<br/> | ||
| + | continue from experiment: Ligation<br/> | ||
| + | Project name: inducible promoter for pbd<br/> | ||
| + | samples stored in incubator | ||
| + | |||
| + | ===PCR=== | ||
| + | |||
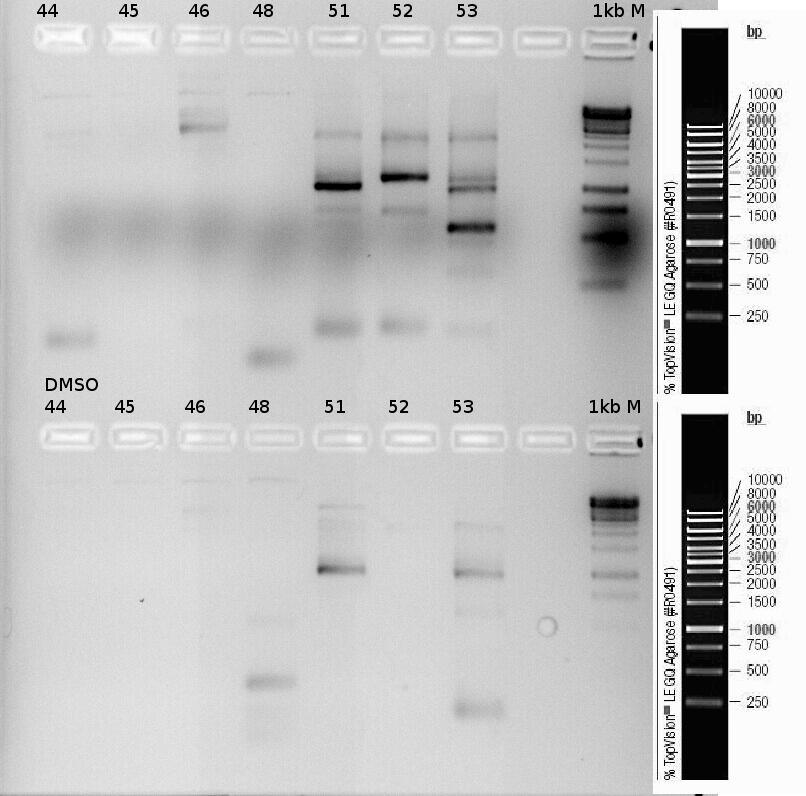
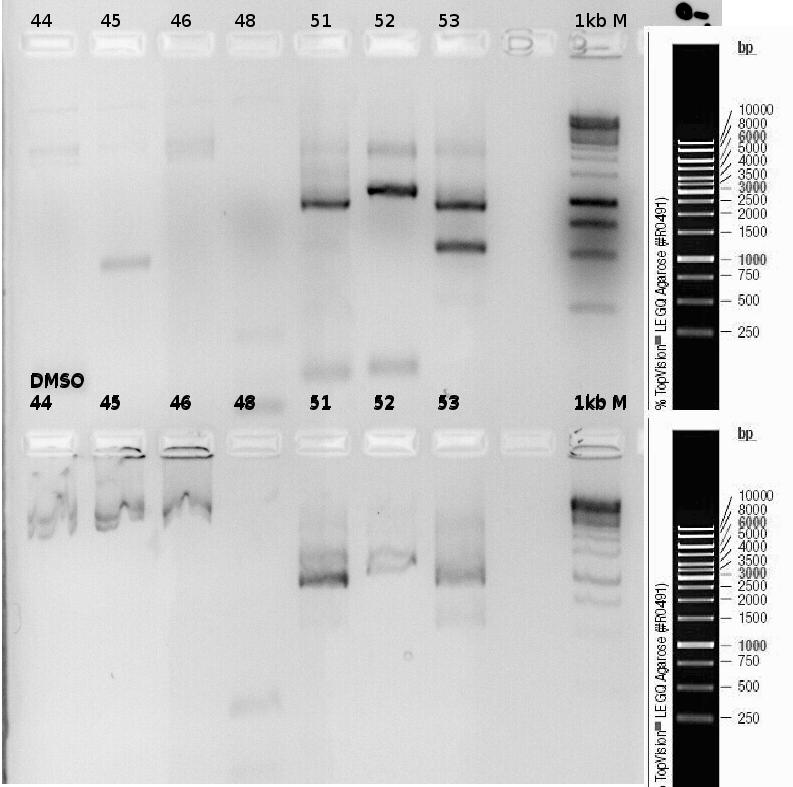
| + | '''Investigators: Rüdiger''' | ||
| + | |||
| + | PCR of precipitator. | ||
| + | |||
| + | Primer: | ||
| + | *p44 | ||
| + | *p45 | ||
| + | *p46 | ||
| + | *p48 | ||
| + | *51 | ||
| + | *52 | ||
| + | *53 | ||
| + | |||
| + | Primer: | ||
| + | *p54 | ||
| + | *p47 | ||
| + | |||
| + | {| cellpadding="10" cellspacing="0" border="1" | ||
| + | |name | ||
| + | |primer | ||
| + | |primer | ||
| + | |- | ||
| + | |44 | ||
| + | |p44 | ||
| + | |p54 | ||
| + | |- | ||
| + | |45 | ||
| + | |p45 | ||
| + | |p54 | ||
| + | |- | ||
| + | |46 | ||
| + | |p46 | ||
| + | |p54 | ||
| + | |- | ||
| + | |48 | ||
| + | |p48 | ||
| + | |p47 | ||
| + | |- | ||
| + | |51 | ||
| + | |p51 | ||
| + | |p54 | ||
| + | |- | ||
| + | |52 | ||
| + | |p52 | ||
| + | |p54 | ||
| + | |- | ||
| + | |53 | ||
| + | |p53 | ||
| + | |p54 | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[File:Freiburg_2011_Testdigest1.jpg|500px]] | ||
| + | |||
| + | [[File:Freiburg_2011_tesdigest2.jpg|500px]] | ||
| + | |||
| + | [[File:Freiburg_2011_testdigest3.jpg|500px]] | ||
| - | |||
{{:Team:Freiburg/Templates/footer}} | {{:Team:Freiburg/Templates/footer}} | ||
Latest revision as of 01:08, 22 September 2011
 "
"
 Contact
Contact