Team:Wageningen UR/Safety
From 2011.igem.org
(→Safety) |
(→Safety) |
||
| (23 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <html> | |
| - | = | + | <head> |
| + | <style type="text/css"> | ||
| - | { | + | ul li a.currentlinktop7 { |
| - | + | color: #63a015 !important; | |
| + | } | ||
| - | __NOTOC__ | + | </style> |
| + | </head> | ||
| + | <bod> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | {{:Team:Wageningen_UR/Templates/Header}} | ||
| + | {{:Team:Wageningen_UR/Templates/NavigationTop1}} | ||
| + | == Safety == | ||
| + | {{:Team:Wageningen_UR/Templates/Style | text= __NOTOC__ | ||
=== Biosafety Considerations === | === Biosafety Considerations === | ||
| Line 23: | Line 34: | ||
====== '' A. nidulans '' ====== | ====== '' A. nidulans '' ====== | ||
| - | The filamentous fungus ''Aspergillus nidulans'' (strain pyrG89 argB2 pabaB22 nkuATargB riboB2, ordered from FGSC; A1147) is | + | The filamentous fungus ''Aspergillus nidulans'' (strain pyrG89 argB2 pabaB22 nkuATargB riboB2, ordered from FGSC; A1147) is capable of producing spores. Though the route of infection should be effective, according to Kim et al., disease caused by the micro-organsim is rarely seen in healthy persons (1997 Jun; [[#References| Jump to references]]). Correspondingly, the Dutch ‘Regeling Genetisch Gemodificeerde Organismen' (Regulation of Genetically Modified Organisms) classified ''A. nidulans'' as Biosafety level (BSL) 1 and allows genetical modifications when it's performed in a ML-II lab. |
| - | Project developments may require work with ''Aspergillus niger'' in addition to ''A. nidulans''. This organism is also classified as BSL 1 | + | Project developments may require work with ''Aspergillus niger'' in addition to ''A. nidulans''. This micro-organism is also classified as BSL 1 and is therefore subject to the same safety considerations as ''A. nidulans''. |
| + | |||
| + | |||
| + | [[File:schimmel_op_brood.jpg|200px]] | ||
| + | |||
| + | '''Fig.1.''' ''Aspergillus nidulans is a green mould that is a common contaminant of starchy foods, such as bread.'' | ||
| Line 31: | Line 47: | ||
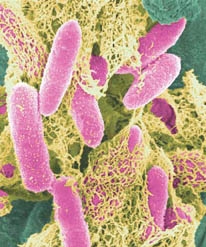
The ''Escherichia coli'' TOP10 strain used in this project is a derivative of the non-pathogenic K-12 laboratory strain. The likelihood of a human becoming infected is therefore low. The most probable route of transmission would occur by accidental ingestion, though the bacterium’s inability to be retained in the human gut decreases the chance of it to develop disease in humans (EPA, 2011; [[#References|Jump to References]]). Mainly for these reasons, this organism is also classified as BSL 1. | The ''Escherichia coli'' TOP10 strain used in this project is a derivative of the non-pathogenic K-12 laboratory strain. The likelihood of a human becoming infected is therefore low. The most probable route of transmission would occur by accidental ingestion, though the bacterium’s inability to be retained in the human gut decreases the chance of it to develop disease in humans (EPA, 2011; [[#References|Jump to References]]). Mainly for these reasons, this organism is also classified as BSL 1. | ||
| + | |||
| + | |||
| + | [[File:E-coli-in-color.jpg]] | ||
| + | |||
| + | '''Fig.2.''' ''A microscopic picture of E. coli cells. The length of a cell is about 2 micrometers. The colours are not real.'' | ||
| Line 36: | Line 57: | ||
| - | ====== Track ‘n Trace ====== | + | ====== Fungal Track ‘n Trace ====== |
The main hazard in this project is the accidental release of genetically modified spores into the environment. This could potentially occur during transportation if samples are not properly contained. Because the life cycle of ''Aspergillus'' contains a sporulation phase, it is subject to a higher risk of being released into the environment than organisms which replicate through simple cell division. | The main hazard in this project is the accidental release of genetically modified spores into the environment. This could potentially occur during transportation if samples are not properly contained. Because the life cycle of ''Aspergillus'' contains a sporulation phase, it is subject to a higher risk of being released into the environment than organisms which replicate through simple cell division. | ||
| Line 68: | Line 89: | ||
===== Specific Hazards ===== | ===== Specific Hazards ===== | ||
| - | ====== Track ‘n Trace ====== | + | ====== Fungal Track ‘n Trace ====== |
The Track ‘n Trace project involves modifying the leucine metabolism of ''A. nidulans'' and using metabolic intermediates to regulate the expression of fluorescent proteins. We do not expect these modifications to increase the pathogenicity or fitness of the ''A. nidulans'' strain. Therefore, we do not anticipate any additional safety hazards going beyond those inherent to working with ''A. nidulans'' in a laboratory environment. | The Track ‘n Trace project involves modifying the leucine metabolism of ''A. nidulans'' and using metabolic intermediates to regulate the expression of fluorescent proteins. We do not expect these modifications to increase the pathogenicity or fitness of the ''A. nidulans'' strain. Therefore, we do not anticipate any additional safety hazards going beyond those inherent to working with ''A. nidulans'' in a laboratory environment. | ||
| Line 105: | Line 126: | ||
When there is (uncertainty about) relevant biosafety information, it would be good to indicate this in the Design Notes of the Part Design page of the RSBP. Furthermore it should become clear from the introduction on the Main Page whether the BioBrick part produces a biohazardous product or the system might be harmful in total and if a subpart falls out of the superpart. Because effects of release out of the containment can be unpredictable, biosafety information wouldn’t be at the right place on the page Hard Information. A team might have designed a part without producing and validating it. If this part is then used in a new system and turns out to be hazardous during the construction this should also be noticed under User Reviews at the Experience page. For this purpose, the team that used the BioBrick design should be able to edit this page. | When there is (uncertainty about) relevant biosafety information, it would be good to indicate this in the Design Notes of the Part Design page of the RSBP. Furthermore it should become clear from the introduction on the Main Page whether the BioBrick part produces a biohazardous product or the system might be harmful in total and if a subpart falls out of the superpart. Because effects of release out of the containment can be unpredictable, biosafety information wouldn’t be at the right place on the page Hard Information. A team might have designed a part without producing and validating it. If this part is then used in a new system and turns out to be hazardous during the construction this should also be noticed under User Reviews at the Experience page. For this purpose, the team that used the BioBrick design should be able to edit this page. | ||
| - | The indications of biosafety could even be more generalized when at the BioBrick part entry page of the RSBP there would be requested to enter these indications. This, by filling in two extra text entry box(es) headed with, the notions on safety indicated by iGEM | + | The indications of biosafety could even be more generalized when at the BioBrick part entry page of the RSBP there would be requested to enter these indications. This, by filling in two extra text entry box(es) headed with, the notions on safety indicated by iGEM: |
| - | + | :*Foreseen BioBrick Part or System/Device risk(s) and | |
| - | + | :*‘Chassis enhancement’ | |
| - | or by marking | + | or by marking a ‘No relevant biosafety issue’ checkbox (2011; [[#References|Jump to References]]). They could also come out better if the Main Page of the BioBrick part had a Biosafety category, that would add relevant biosafety information from the entry page automatically. |
| Line 128: | Line 149: | ||
==== 4. Improvements on safety ==== | ==== 4. Improvements on safety ==== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 157: | Line 163: | ||
==== References ==== | ==== References ==== | ||
| + | |||
| + | |||
| + | Kim M, Shin JH, Suh SP, Ryang DW, Park CS, Kim C, Kook H, Kim J. '''Aspergillus nidulans infection in a patient with chronic granulomatous disease'''. J Korean Med Sci. 1997 Jun;12(3):244-248. URL: http://synapse.koreamed.org/DOIx.php?id=10.3346%2Fjkms.1997.12.3.244. A small fragment was used. Copyright © 1997 The Korean Academy of Medical Sciences. | ||
| + | :[[#Pathogenicity of host organisms|Return]] | ||
EPA(U.S. Environmental Protection Agency). (2011, January Monday). Escherichia coli K-12 TSCA Section 5(h)(4) Exemption: Final Decision Document. Retrieved July 2011, from Biotechnology Program under the Toxic Substances Control Act (TSCA): | EPA(U.S. Environmental Protection Agency). (2011, January Monday). Escherichia coli K-12 TSCA Section 5(h)(4) Exemption: Final Decision Document. Retrieved July 2011, from Biotechnology Program under the Toxic Substances Control Act (TSCA): | ||
http://www.epa.gov/biotech_rule/pubs/fra/fd004.htm | http://www.epa.gov/biotech_rule/pubs/fra/fd004.htm | ||
| - | :[[#Pathogenicity of host organisms| Return]] | + | :[[#Pathogenicity of host organisms|Return]] |
Latest revision as of 21:29, 18 August 2011
 "
"