Team:XMU-China/Project/Background
From 2011.igem.org
(Difference between revisions)
(→reference) |
|||
| (5 intermediate revisions not shown) | |||
| Line 18: | Line 18: | ||
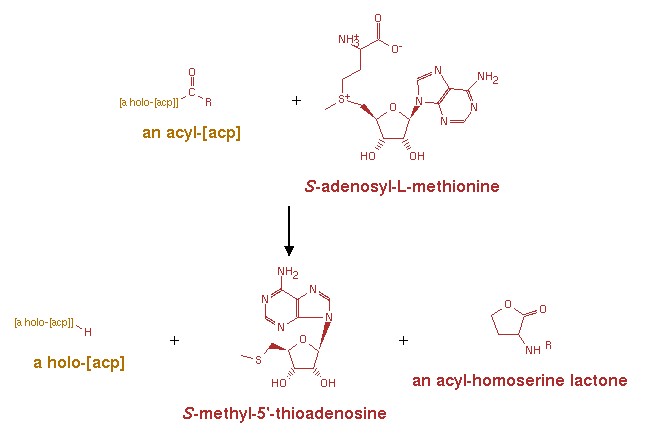
An acyl-[acyl-carrier-protein] + S-adenosyl-L-methionine = [acyl-carrier-protein] + S-methyl-5'-thioadenosine + an N-acyl-L-homoserine lactone.[8] | An acyl-[acyl-carrier-protein] + S-adenosyl-L-methionine = [acyl-carrier-protein] + S-methyl-5'-thioadenosine + an N-acyl-L-homoserine lactone.[8] | ||
| - | + | [[Image:XMU_China_135.jpg|left|frame|Figure 1:The reaction mechanism of the synthesis of AHL.]] | |
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| - | |||
====''' LuxR: Transcriptional activator protein luxR'''==== | ====''' LuxR: Transcriptional activator protein luxR'''==== | ||
| Line 31: | Line 34: | ||
AHL is a kind of signaling molecule involved in bacterial quorum sensing.[2] In Vibrio fischeri, AHL binds to the protein product of the LuxR gene and activates it. The C-terminal domain of activated LuxR relieves the repression exerted by H-NS nucleoid proteins that bind to the promoters of LuxR, LuxI and the LuxCDABEG operon, as well as to A-T-rich stretches within that operon and other genomic regions. The product of LuxI catalyses the synthesis of AHL. Thus, AHL acts as an autoinducer. Transcription of the LuxCDABEG operon results in luminescence due to the expression of LuxA and LuxB, which form a protein known as a luciferase and the expression of LuxC, D, E, and G, which are involved in the synthesis of the luciferase's substrate, tetradecanal.[11] | AHL is a kind of signaling molecule involved in bacterial quorum sensing.[2] In Vibrio fischeri, AHL binds to the protein product of the LuxR gene and activates it. The C-terminal domain of activated LuxR relieves the repression exerted by H-NS nucleoid proteins that bind to the promoters of LuxR, LuxI and the LuxCDABEG operon, as well as to A-T-rich stretches within that operon and other genomic regions. The product of LuxI catalyses the synthesis of AHL. Thus, AHL acts as an autoinducer. Transcription of the LuxCDABEG operon results in luminescence due to the expression of LuxA and LuxB, which form a protein known as a luciferase and the expression of LuxC, D, E, and G, which are involved in the synthesis of the luciferase's substrate, tetradecanal.[11] | ||
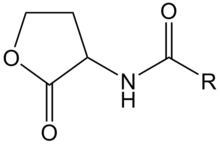
| - | + | [[Image:XMU_China_136.jpg|left|frame|Figure 2: General chemical structure of an N-acyl homoserine lactone.]] | |
| - | |||
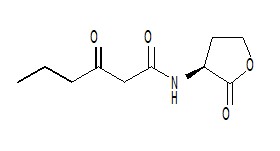
| - | + | [[Image:XMU_China_137.jpg|left|frame|Figure 3: N-Hexanoyl-L-homoserine lactone.]] | |
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| - | |||
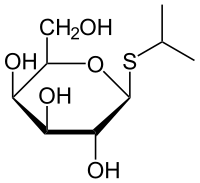
====''' IPTG: Isopropyl β-D-1-thiogalactopyranoside'''==== | ====''' IPTG: Isopropyl β-D-1-thiogalactopyranoside'''==== | ||
| Line 43: | Line 48: | ||
This compound is used as a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon. Many regulatory elements of the lac operon are used in inducible recombinant protein systems.[14] | This compound is used as a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon. Many regulatory elements of the lac operon are used in inducible recombinant protein systems.[14] | ||
| - | + | [[Image:XMU_China_138.jpg|left|frame|Figure 4 General chemical structure of IPTG.]] | |
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| - | |||
iGEM-Team XMU-China has designed a series of circuits driven by The PLlac 0-1 promoter (BBa_R0011). IPTG was used as an inducer to activate these circuits. | iGEM-Team XMU-China has designed a series of circuits driven by The PLlac 0-1 promoter (BBa_R0011). IPTG was used as an inducer to activate these circuits. | ||
| Line 67: | Line 75: | ||
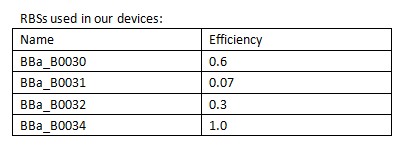
In order to control the expression of the killer protein ccdB, our team designed a series of bacteria population-control devices using RBSs with different strength. The cell growth and fluorescent curves corresponding to different RBSs illustrate that the bacteria population was successfully controlled at different cell densities. | In order to control the expression of the killer protein ccdB, our team designed a series of bacteria population-control devices using RBSs with different strength. The cell growth and fluorescent curves corresponding to different RBSs illustrate that the bacteria population was successfully controlled at different cell densities. | ||
| - | + | [[Image:XMU_China_139.jpg|left|frame|Table 1 RBSs of different strength.]] | |
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| - | |||
===Lux pR=== | ===Lux pR=== | ||
| Line 76: | Line 87: | ||
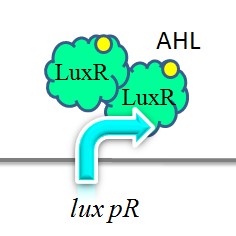
iGEM-Team XMU-China has successfully strengthened the expression of lux pR by mutating its DNA sequence. Three mutants, IR-3, IR-5, IR-3/5, were obtained by site-directed mutagenesis using 3-step PCR method. | iGEM-Team XMU-China has successfully strengthened the expression of lux pR by mutating its DNA sequence. Three mutants, IR-3, IR-5, IR-3/5, were obtained by site-directed mutagenesis using 3-step PCR method. | ||
| - | + | [[Image:XMU_China_140.jpg|left|frame|Figure 5: Two molecules of LuxR protein form a complex with two molecules of the signalling compound homoserine lactone (HSL). This complex binds to a palindromic site on the promoter, increasing the rate of transcriptionTwo molecules of LuxR protein form a complex with two molecules of the signalling compound homoserine lactone (HSL). This complex binds to a palindromic site on the promoter, increasing the rate of transcription.]] | |
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| - | |||
===IR-GFP=== | ===IR-GFP=== | ||
| Line 84: | Line 98: | ||
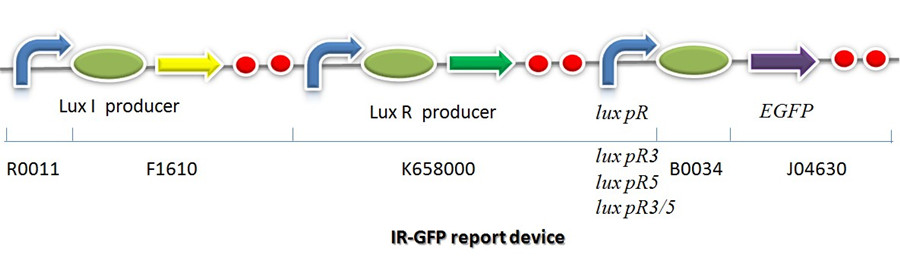
IR-GFP is a series of report devices designed for testing the performance of lux R promoters before and after mutagenesis. These four IR-GFP report devices have been transformed into E.coli string BL21 separately. These devices produced greenish tint visible by naked eyes when induced by IPTG. We measured and compared their florescent intensities at steady state. As the only difference between the four devices is Lux R promoter, the efficiency of the four Lux R promoters could be defined. | IR-GFP is a series of report devices designed for testing the performance of lux R promoters before and after mutagenesis. These four IR-GFP report devices have been transformed into E.coli string BL21 separately. These devices produced greenish tint visible by naked eyes when induced by IPTG. We measured and compared their florescent intensities at steady state. As the only difference between the four devices is Lux R promoter, the efficiency of the four Lux R promoters could be defined. | ||
| - | + | [[Image:XMU_China_141beta.jpg|900px|left|frame|Figure 6: Promoter lux pR strength testing circuit.]] | |
| + | |||
| + | ==Reference== | ||
| + | [1] Koch B, Liljefors T, Persson T , Nielsen J, Kjelleberg S, Givskov M. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors[J].Microbiology , 2005, 151 (11): 3589-3602. | ||
| + | |||
| + | [2] Kumari A, Pasini P, Deo S. K., Flomenhoft D, Shashidhar S, Daunert S. Biosensing Systems for the Detection of Bacterial Quorum Signaling Molecules[J]. Analytical Chemistry,2005, 78 (22): 7603–7609. | ||
| + | |||
| + | [3] Eberhard A, Burlingame A. L, Eberhard C, Kenyon G. L, Nealson K. H,Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase[J]. Biochemistry ,1981,20 (9): 2444–2449. | ||
| + | |||
| + | [4] Gerdes K. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress[J]. J. Bacteriol,2000,182 (3): 561–721. | ||
| + | |||
| + | [5]Philippe B,Martine C.Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes[J].Molecular Biology,1992,226(3):735-745. | ||
| + | |||
| + | [6]Devine J.H,Shadel G.S,Baldwin T.O. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proceedings of the National Academy of Sciences.1989.86(15):5688-5692. | ||
| + | |||
| + | [7]Miki T,Yoshioka K,Horiuchi T. Control of cell division by sex factor F in Escherichia coli. I. The 42.84-43.6 F segment couples cell division of the host bacteria with replication of plasmid DNA. Molecular Biology,1984,177(4):605-625. | ||
| + | |||
| + | [8] Hanzelka BL, Greenberg E. Quorum sensing in Vibrio fischeri: evidence that S-adenosyl methionine is the amino acid substrate for autoinducer synthesis[J]. Journal of Bacteriology, 1996, 178(17): 5291-5294. | ||
| + | |||
| + | [9] Urbanowski M, Lostroh C, Greenberg E. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein[J]. Journal of Bacteriology, 2004, 186(3): 631-637. | ||
| + | |||
| + | [10] Hansen LH, Knudsen S, Sorensen SJ . The effect of the lacY gene on the induction of IPTG inducible promoters, studied in Escherichia coli and Pseudomonas fluorescens. Curr microbiol,1998, 36 (6): 341–345. | ||
| + | |||
| + | [11]Bernard P,Couturier M. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. Molecular Biology,1992,226:735-745. | ||
| + | |||
| + | [12]Semit M.H,Duin J. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Molecular Biology,1990,87(19):7668-7672. | ||
| + | |||
| + | [13] Baldwin T, Devine JH, Heckel, RC, Lin, JW, Shadel GS. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence[J]. Journal of Bioluminescence and Chemiluminescence, 1989, 4(1): 326-341. | ||
| + | |||
| + | [14] You L, Cox RS, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428(6985): 868-871. | ||
| + | |||
| + | [15] http://en.wikipedia.org/wiki/N-Acyl_homoserine_lactone | ||
| + | |||
| + | [16] http://scholarworks.umass.edu/dissertations/AAI3397713/ | ||
| + | |||
| + | [17] http://en.wikipedia.org/wiki/Toxin-antitoxin_system | ||
| + | |||
| + | [18] http://www.sciencedirect.com/science/article/pii/002228369290629X | ||
| + | |||
| + | [19] http://scholarworks.umass.edu/dissertations/AAI3397713/ | ||
| + | |||
| + | [20] http://www.uniprot.org/uniprot/P12746 | ||
| + | |||
| + | [21] http://www.uniprot.org/uniprot/P12747 | ||
| + | |||
| + | [22] http://www.uniprot.org/uniprot/P62554 | ||
| + | |||
| + | [23] http://www.nottingham.ac.uk/quorum/what2.htm | ||
| + | |||
| + | [24] http://www.humancyc.org/META/NEW-IMAGE?type=REACTION&object=2.3.1.184-RXN | ||
| + | |||
| + | [25] http://en.wikipedia.org/wiki/IPTG | ||
| + | |||
| + | [26] http://www.uniprot.org/uniprot/P62554 | ||
| - | + | [27] http://en.wikipedia.org/wiki/Ribosomal_binding_site | |
Latest revision as of 02:18, 29 October 2011
 "
"