Team:XMU-China/Project/Appoach
From 2011.igem.org
(Difference between revisions)
(Created page with "{{:Team:XMU-China/Hidden}} {{:Team:XMU-China/List}} In December 2010, we began thinking about project for 2011 .We tried to get inspiration from materials provided by iGEM teams...") |
|||
| (7 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{:Team:XMU-China/List}} | {{:Team:XMU-China/List}} | ||
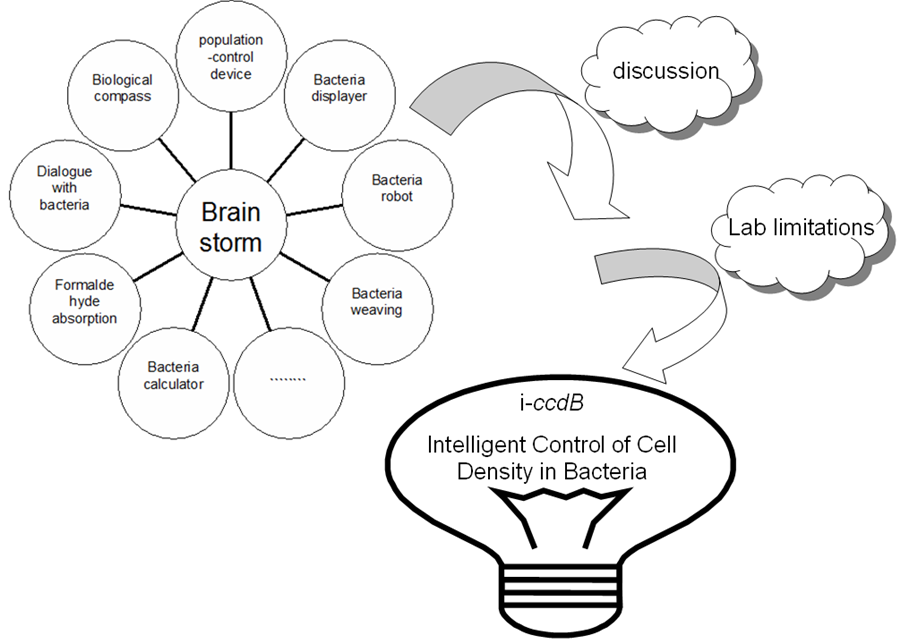
| - | In December 2010, we began thinking about project for 2011 .We tried to get inspiration from materials provided by iGEM teams in past three years. And we put forward many ideas. For example, we could construct a kind of E.coli which can decompose and detect carbinol harming human body. Another example is that we try to make E.coli have magneto taxis. We collected papers about those ideas | + | In December 2010, we began thinking about project for 2011 iGEM .We tried to get inspiration from materials provided by iGEM teams in past three years. And we put forward many ideas. For example, we could construct a kind of E.coli which can decompose and detect carbinol harming human body. Another example is that we try to make E.coli have magneto taxis. We collected papers about those ideas. |
| - | + | One day, our instructor had Pu’er tea in his office and said to us that the tea wasn’t bitter at all. Instead, it tasted sweet because it had been treated with some special methods. One of team member found it an interesting topic. So she referred to some books for information about the fermentation of the Pu’er tea. She found that the concentration of bacteria in the fermentation process was the most important factor of determining the taste of the tea. The prevailing method used for controlling the concentration of bacteria is by controlling the concentration of the medium. We came up with the idea that fermentation technology can be simplified if bacteria can maintain at the optimum concentration by itself. | |
| + | |||
| + | We are inspired by a paper published on natrue[3]. And we thought that we could also use the method of synthetic biology to solve the problems which could be solved by traditional biology method and perhaps we could do even better and make great improvements. So we came up with the idea of making a series bacteria population-control devices. | ||
| + | |||
| + | |||
| + | [[Image:XMU_China_134beta.jpg|left|Figure 1: From brainstorm to our project i-''ccdB''|frame|Figure 1: From brainstorm to our project i-''ccdB''.]] | ||
| + | |||
| + | <html> | ||
| + | <img src="http://partsregistry.org/wiki/images/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| - | |||
== Design == | == Design == | ||
| Line 21: | Line 29: | ||
3.using the method of site-directed mutagenesis to modify the promoter lux pR,changing its efficiency | 3.using the method of site-directed mutagenesis to modify the promoter lux pR,changing its efficiency | ||
| - | 4. performing these | + | 4. performing these circuits |
== Aims == | == Aims == | ||
| - | Bacteria concentration has significant impact on production of fermentation product. Product concentration is proportional with bacteria concentration at suitable special growth rate. The primary metabolite production is the case, such as amino acid and vitamin. However, for secondary metabolite production, such as antibiotic, the special growth rate should not be too high. Otherwise, it would generate negative impacts, such as fast consumption of nutrient substance, significant change of culture media, accumulation of toxic substance in culture media and significant decline of | + | Bacteria concentration has significant impact on production of fermentation product. Product concentration is proportional with bacteria concentration at suitable special growth rate. The primary metabolite production is the case, such as amino acid and vitamin. However, for secondary metabolite production, such as antibiotic, the special growth rate should not be too high. Otherwise, it would generate negative impacts, such as fast consumption of nutrient substance, significant change of culture media, accumulation of toxic substance in culture media and significant decline of OD, which would alter the metabolic pathway of bacteria. We aimed at constructing a series of bacteria can maintain at the different concentration by itself. We hope that our work can introduce a new way which controls the concentration of bacteria for fermentation process control technology. |
| - | |||
== The Final Product == | == The Final Product == | ||
The device is designed to build a programmed bacterial death circuit, which is based on the quorum sensing system of Vibrio fischeri. The LuxI protein synthesizes a small, diffusible acyl-homoserinelactone (AHL) signaling molecule. The AHL accumulates as the cell density increases. At sufficiently high concentrations, it binds the LuxR, which induces the expression of the killer gene ccdB under the control of a promoter lux pR. Sufficiently high levels of CcdB which is a bacterial toxin that targets DNA gyrase cause cell death. Low cell density doesn’t have the ability to produce sufficient LuxR/AHL complex to activate the promoter lux pR. The programmed death circuit ends and the cell density increases. When the cells reach a certain concentration, the death circuit is restarted. Back and forth, the programmed death is achieved in the dynamic process of growth and death. In that way, the bacteria population is programmed to maintain one certain cell density. In order to control the expression of the killer protein ccdB, we designed a series of bacteria population-control devices using RBSs with different efficiency. The cell growth and fluorescent curves corresponding to different RBSs illustrate that the bacteria population was successfully controlled at different cell densities. | The device is designed to build a programmed bacterial death circuit, which is based on the quorum sensing system of Vibrio fischeri. The LuxI protein synthesizes a small, diffusible acyl-homoserinelactone (AHL) signaling molecule. The AHL accumulates as the cell density increases. At sufficiently high concentrations, it binds the LuxR, which induces the expression of the killer gene ccdB under the control of a promoter lux pR. Sufficiently high levels of CcdB which is a bacterial toxin that targets DNA gyrase cause cell death. Low cell density doesn’t have the ability to produce sufficient LuxR/AHL complex to activate the promoter lux pR. The programmed death circuit ends and the cell density increases. When the cells reach a certain concentration, the death circuit is restarted. Back and forth, the programmed death is achieved in the dynamic process of growth and death. In that way, the bacteria population is programmed to maintain one certain cell density. In order to control the expression of the killer protein ccdB, we designed a series of bacteria population-control devices using RBSs with different efficiency. The cell growth and fluorescent curves corresponding to different RBSs illustrate that the bacteria population was successfully controlled at different cell densities. | ||
| + | |||
| + | |||
| + | ==Reference== | ||
| + | [1] http://en.wikipedia.org/wiki/Pu-erh_tea | ||
| + | |||
| + | [2] Baldwin T, Devine JH, Heckel, RC, Lin, JW, Shadel GS. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence[J]. Journal of Bioluminescence and Chemiluminescence, 1989, 4(1): 326-341. | ||
| + | |||
| + | [3] You L, Cox RS, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428(6985): 868-871. | ||
| + | |||
| + | [4]Philippe B,Martine C.Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes[J].Molecular Biology,1992,226(3):735-745. | ||
Latest revision as of 03:59, 29 October 2011
 "
"