Team:Korea U Seoul/Project/Design
From 2011.igem.org
(Difference between revisions)
(→Project Design) |
(→Hydrocarbon detection) |
||
| (6 intermediate revisions not shown) | |||
| Line 37: | Line 37: | ||
*Lux genes | *Lux genes | ||
:- The genes from bioluminescence operons have been identified, and we use some structural genes (''luxC'', ''D'', and ''E'' genes). They code for the polypeptides of the fatty acid reductase system responsible for synthesis of the fatty aldehyde substrate. | :- The genes from bioluminescence operons have been identified, and we use some structural genes (''luxC'', ''D'', and ''E'' genes). They code for the polypeptides of the fatty acid reductase system responsible for synthesis of the fatty aldehyde substrate. | ||
| - | :- There are 5 lux genes that we used. ''lux A'', ''B'', ''C'', ''D'', and ''E'' were used. ''lux A'' and ''lux B'' codes Luciferase alpha and beta subunit respectively. ''lux C'' codes Reductase, ''lux D'' codes Acyl-transferase, and ''lux E'' codes Synthetase. Luciferase alpha and beta subunit function is catalysis of the bioluminescence reaction(FMNH2 + O2 + aldehyde -> light). Reductase's function is NADPH-dependent reduction of activated fatty acyl groups to aldehyde. Acyl-transferase's function is generation of fatty acids(tetradecanoic acid) for the luminescence system. Lastly, Synthetase's function is ATP-dependent activation of fatty acids. | + | :- There are 5 lux genes that we used. ''lux A'', ''B'', ''C'', ''D'', and ''E'' were used. ''lux A'' and ''lux B'' codes Luciferase alpha and beta subunit respectively. ''lux C'' codes Reductase, ''lux D'' codes Acyl-transferase, and ''lux E'' codes Synthetase. Luciferase alpha and beta subunit function is catalysis of the bioluminescence reaction(FMNH2 + O2 + aldehyde -> light). Reductase's function is NADPH-dependent reduction of activated fatty acyl groups to aldehyde. Acyl-transferase's function is generation of fatty acids(tetradecanoic acid) for the luminescence system. Lastly, Synthetase's function is ATP-dependent activation of fatty acids. |
| - | [[File: | + | [[File:lux.jpg|thumb|Bioluminescence|450px|left]] |
| - | + | [[File:arrows.jpg|thumb|Manipulation of Lux genes|450px|right]] | |
| - | *We are currently having difficulty in detecting the final product, | + | |
| - | :- Detection of | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == '''Hydrocarbon detection''' == | ||
| + | |||
| + | *We are currently having difficulty in detecting the final product, C13. | ||
| + | :- Detection of C13 by TLC is difficult. It is not yet providing good enough results. Apparently, this is due to the fact that saturated hydrocarbons don't react with any other compounds. | ||
:- By incorporating BBa_K32599 in luxCDEG, we may be able to detect hydrocarbon. | :- By incorporating BBa_K32599 in luxCDEG, we may be able to detect hydrocarbon. | ||
Latest revision as of 00:06, 6 October 2011
Project Design
- E.coli K27 strains
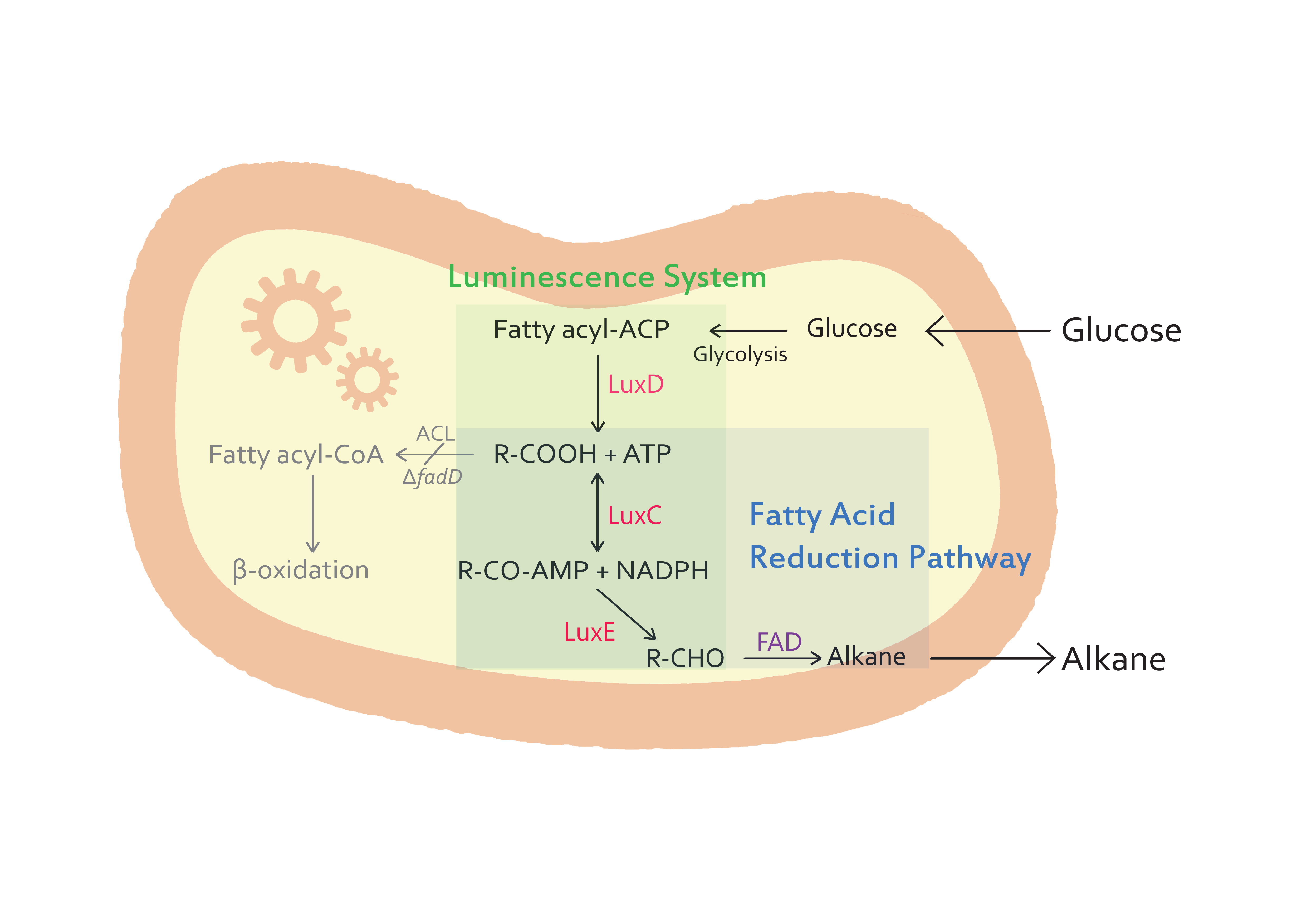
- - The purpose of our team is to synthesize alkanes from microorganisms. E.coli K27 is a suitable host for the production of alkanes because it is a FadD mutant(△fadD).
- - In our step of alkanes synthesis, the fatty acids are important intermediates. Commonly, E.coli cells contain a single acyl-CoA synthetase, which activates the conversion of free fatty acid to acyl-CoA thioester. However, E.coli K27, a FadD mutant, lacks acyl-CoA synthetase activity, which prevents substrate or product degradation by the host. So, E.coli K27 accumulates fatty acids inside the cell, and finally we can get more alkanes than other E.coli strains.
- Two-carbon compounds and fatty acids as carbon sources
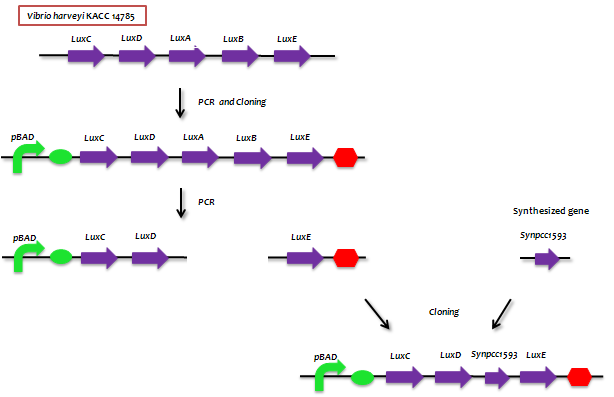
- Lux genes
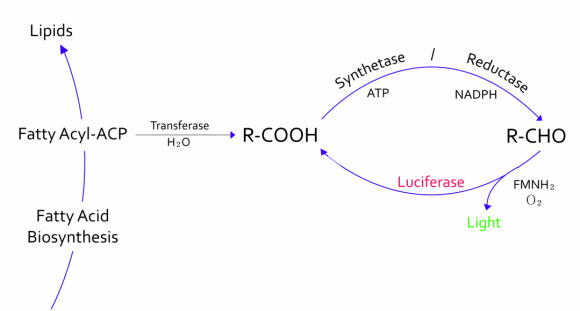
- - The genes from bioluminescence operons have been identified, and we use some structural genes (luxC, D, and E genes). They code for the polypeptides of the fatty acid reductase system responsible for synthesis of the fatty aldehyde substrate.
- - There are 5 lux genes that we used. lux A, B, C, D, and E were used. lux A and lux B codes Luciferase alpha and beta subunit respectively. lux C codes Reductase, lux D codes Acyl-transferase, and lux E codes Synthetase. Luciferase alpha and beta subunit function is catalysis of the bioluminescence reaction(FMNH2 + O2 + aldehyde -> light). Reductase's function is NADPH-dependent reduction of activated fatty acyl groups to aldehyde. Acyl-transferase's function is generation of fatty acids(tetradecanoic acid) for the luminescence system. Lastly, Synthetase's function is ATP-dependent activation of fatty acids.
Hydrocarbon detection
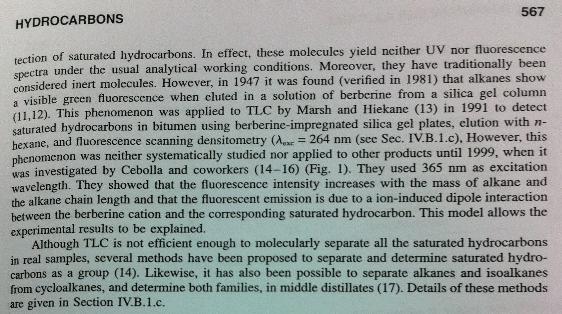
- We are currently having difficulty in detecting the final product, C13.
- - Detection of C13 by TLC is difficult. It is not yet providing good enough results. Apparently, this is due to the fact that saturated hydrocarbons don't react with any other compounds.
- - By incorporating BBa_K32599 in luxCDEG, we may be able to detect hydrocarbon.
- Summary
- - Thin-layer chromatography (TLC) is a particularly well adapted technique for separating liquid or soluble hydrocarbon-containing samples and, in general, complex or dirty samples, all of which include fractions or impurities that can contaminate the stationary phase.
- - One of the most important advantages of TLC over HPLC for hydrocarbon analysis is the possibility of scanning the whole sample without no prior sample preparation steps.
- Summary
- - TLC-Densitometry has seldom been applied to hydrocarbon analysis because of difficulties in the detection of saturated hydrocarbons.
- - Alkanes show a visible green fluorescence when cluted in a solution of berberine from a silica gel column. This phenomenon was applied to TLC to detect saturated hydrocarbons in bitumen using berberine-impregnated silica gel plates, elution with n-hexane, and fluorescence scanning densitometry.
- Unfortunately, we do not have berberine currently, and therefore cannot use it for hydrocarbon detection.
Contact : |
synbiogroup@googlegroups.com |
 |
|
|
|
 "
"