Team:Kyoto/Capture
From 2011.igem.org
Grassfield (Talk | contribs) (→Suitable wavelength of the light to lure drosophilas) |
Grassfield (Talk | contribs) (→Experiment 2 --- Assay of drosophila's phototaxis to the light emitted by E.coli) |
||
| (132 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
= '''Project Capture''' = | = '''Project Capture''' = | ||
| - | + | =='''1. Introduction/Background'''== | |
| - | + | <div class="week"> | |
| - | == '''Introduction''' == | + | ===Methods to capture insects in the nature=== |
| - | + | [[File:京都ハエ捕りそう.jpg|thumb|left|Fig.1]] | |
| - | + | In the nature, organisms capture and eat insects by various ways. For example, spiders make cobwebs to capture insects. Nepenthes has pitchers which lure and trap insects. ''Pyrearinus termitilluminans'', which makes and lives in a tunnel around the external surface of the anthill of termite Cornitermes cumulansand, emits light in the first week of the rainy season to lure and hunt the termites. | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ===Methods to capture insects in nature=== | + | |
| - | In nature, | + | |
| - | + | ||
===What is the feasible method to capture insects for Carnivorous E. coli?=== | ===What is the feasible method to capture insects for Carnivorous E. coli?=== | ||
| - | [[File:Drawning drosophila.jpg|thumb|right|Fig.2]] | + | [[File:Drawning drosophila.jpg|thumb|right|250px|Fig.2 : Drowning drosophilas]] |
| - | However, the feasible methods to capture insects for Carnivorous E. coli are limited. Because Escherichia coli | + | However, the feasible methods to capture insects for Carnivorous E. coli are limited. Because ''Escherichia coli'' moves very slowly and can only exist in the form of colonies on an agar plate or in liquid culture medium, it cannot exist in the form of complicated shapes. Considering these features of ''E.coli'', one way for Carnivorous E. coli to capture insects is to lure the insects to itself, and bind them on the colonies or drown them in the liquid medium.<br><br> |
| - | + | We noticed that drosophilas sometimes drown in electrophoresis tanks. Hence we are convinced that a liquid medium is a feasible and simple way to capture insects. If ''E.coli'' can lure insects(drosophilas), it can capture them. | |
| - | We | + | |
===Ways to lure insects=== | ===Ways to lure insects=== | ||
| - | There are | + | There are different ways to lure insects: smell, pheromone, and light. |
| - | Smell is | + | Smell is a popular way to attract insects, as seen in some plants. For example, the Rafflesia attracts flies by it's distinctive odor like rotting flesh. Pheromone is a secreted or excreted chemical factor that triggers social responses in members of the same species. It is effective in very low concentration, and there are some pheromones which have different functions: aggregation, alarm, territory, and so on. Light is also a way to lure some kinds of insects by appealing to their phototaxis. Moths and flies have phototaxis. A few species use light to lure insects: ''Cornitermes cumulansand'' and ''Arachnocampa'' use light to hunt insects.<br><br> |
| - | + | ||
| - | Pheromone is a secreted or excreted chemical factor that triggers | + | |
| - | + | ||
| - | Light is | + | |
| - | + | ||
In this study, we focus on using light as the method to lure insects (drosophilas) because of the following reasons: | In this study, we focus on using light as the method to lure insects (drosophilas) because of the following reasons: | ||
| - | # Lighting is a visible action and we think that it is suitable for our purpose to create animal-like E. coli. | + | # Lighting is a visible action and we think that it is suitable for our purpose to create animal-like ''E. coli''. |
| - | # There is an available biobrick which has the function to emit light: BBa_K3225909 created by iGEM 2010 Cambridge Team. | + | # There is an available biobrick which has the function to emit light: <html><a href="http://partsregistry.org/Part:BBa_K325909">BBa_K3225909</a></html> created by iGEM 2010 Cambridge Team. |
| - | ==Light as a method to lure drosophilas== | + | =='''2. Light as a method to lure drosophilas'''== |
| - | ===Drosophila melanogaster=== | + | ===''Drosophila melanogaster''=== |
| - | We chose Drosophila melanogaster as one of | + | We chose ''Drosophila melanogaster'' as one of Carnivorous E. coli’s prey, and our model organism. ''D. melanogaster'' has a positive phototaxis, lives all over Japan, and they are small (the length of its body is only a few millimeters). Besides that, it is one of the popular model organisms and available for us. |
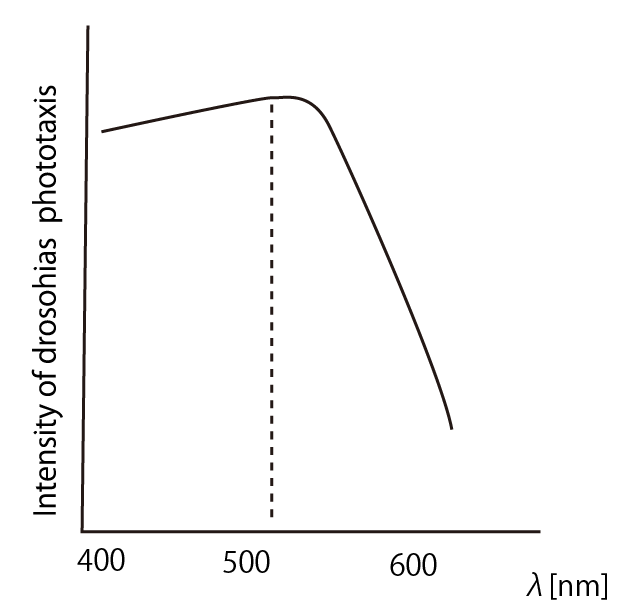
===Suitable wavelength of the light to lure drosophilas=== | ===Suitable wavelength of the light to lure drosophilas=== | ||
| - | + | [[File:Phototaxis colors.PNG|thumb|left|200px|Fig.3]] | |
| + | According to the article <html><a href="http://www.springerlink.com/content/wn464t528n0x1860/">[1]</a></html>, ''drosophila melanogaster'' has a strong positive phototaxis to the light of wavelength shorter than 500nm.(Fig.3)<br><br> | ||
| + | BBa_K325909, one of the biobricks created by iGEM 2010 Cambridge Team, is the lux operon from the ''Viblio fischeri'' which can be used as a L-arabinose --> blue light devise. There is a graph which shows the spectrum of light emitted by ''V. fischeri'' in 2010 Cambridge Team's WIKI(--> <html><a href="https://2010.igem.org/Team:Cambridge/Tools/Lighting">link</a></html>). The peak wavelength is about 500 nm and we can see that the large part of light emitted by BBa_K325909 is in the range of wavelength suitable to lure drosophilas. | ||
| - | + | <div style="width:100%; float:left;"> | |
| + | =='''3. Experiment 1 --- Drosophila's phototaxis in different colors'''== | ||
| + | We made an experiment about drosophila's phototaxis to the lights of different colors(ultraviolet, blue, green, red, and infrared) to show that our assay with a Y-maze is reliable.<br><br> | ||
| + | In the Y-maze, There are two diodes at the two ends of the both sides; one is lighting and the other is not lighting. | ||
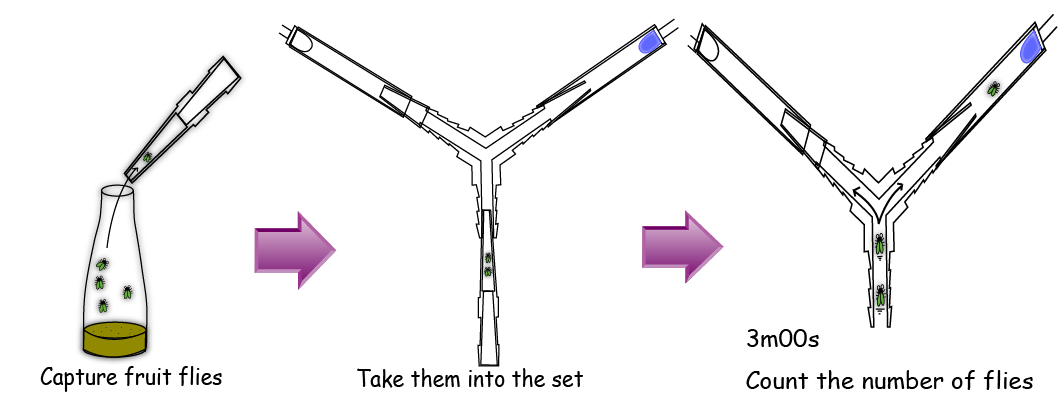
| + | In an assay, we gathered 5 flies with a pooter and put them into the bottom end of the Y maze. After 3 minutes, we counted the number of the flies in each side. (<html><a href="https://2011.igem.org/Team:Kyoto/Measurement">read the materials and methods in detail</a></html>) | ||
| - | + | [[File:Ymaze experiment method.PNG|thumb|center|600px|Fig.4 : The method of the experiment with Y-maze]] | |
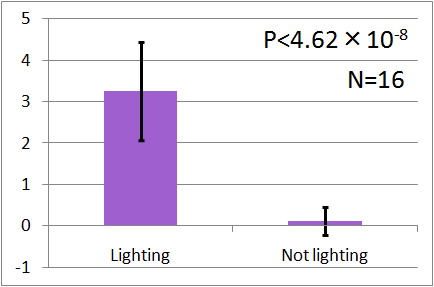
| - | + | The results of these surveys can be summarized as follows (Vertical axis of these graphs indicates the average number of flies. error bars correspond to the standard deviation.) : | |
| - | = | + | <div style="width:100%; float:left"> |
| - | + | [[File:UV graph2.png|thumb|left|200px|Fig.5 : This experiment were performed at UV light.]] | |
| - | + | [[File:Blue graph2.png|thumb|left|200px|Fig.6 : This experiment were performed at blue light.]] | |
| + | [[File:Green graph2.png|thumb|left|200px|Fig.7 : This experiment were performed at green light.]] | ||
| + | [[File:Red graph2.png|thumb|left|200px|Fig.8 : This experiment were performed at red light.]] | ||
| + | [[File:IR graph2.png|thumb|left|200px|Fig.9 : This experiment were performed at infra-red light.]] | ||
| + | </div> | ||
| - | + | In all of these results, the number of flies in light sides is larger than those in the opposite sides. | |
| + | After we run the t test and chi-squared test of those data at a significance level of 5%, | ||
| + | it was revealed that the flies gathered significantly at the LED end under UV light, blue light and green light. On the other hand, when red light was used, the difference between the 2 ends was negligible. This is consistent with the results reported by [2] that drosophilas have a strong positive phototaxis to the light of wavelength shorter than 500nm. It means that our assay of drosophila's phototaxis with Y-maze is a certain assay. | ||
| + | </div> | ||
| - | <div style="width:100%; float:left"> | + | <div style="width:100%; float:left;"> |
| - | + | ||
| - | + | == '''Experiment 2 --- Assay of drosophila's phototaxis to the light emitted by ''E.coli'' ''' == | |
| + | We confirmed that our assay with Y-maze is reliable by experiment 1, and we intended to assay the phototaxis to the light emitted by ''E.coli''. But unfortunately, we couldn't make strong and stable light; some of our ''E.coli'' lighted weakly and others didn't. Besides, our lighting ''E.coli'' lost its light soon. | ||
| - | [[File: | + | <div style="width:100%; float:left"> |
| + | [[File:Lighting E.coli and weak green LED.JPG|thumb|left|320px|Fig.10 : Our lighting ''E.coli'' and weak green LED]] | ||
| + | [[File:Cambridge2010 E.growli.jpg|thumb|left|300px|Fig.11 : Cambridge 2010 Team's lighting ''E.coli''(E.growli) extracted from Cambridge 2010 team's WIKI]] | ||
</div> | </div> | ||
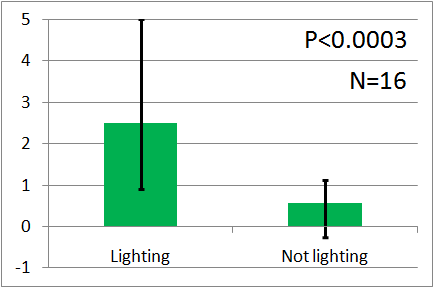
| - | + | [[File:Weakgreen graph2.png|thumb|left|300px|Fig.12 : This experiment were performed at weakgreen light. Vertical axis indicates the average number of flies.]] | |
| - | + | ||
| - | + | ||
| - | + | Instead, we covered a green LED with black film and make it light as weak as our ''E.coli'', and we assayed with the weak green LED by the Y-maze. Here is the result. Unfortunately, the significant difference was not observed. We think that it is because the intensity of the emitted light was too weak. However, as you can see, iGEM 2010 Cambridge team succeeded to make ''E.coli'' emit light and to reinforce the intensity of the light. We think that if our E.coli can emit brighter light like them, the assay will show the significant difference. | |
| - | + | <!-- | |
| - | + | <hr><hr> | |
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | + | As is clear from the figures, the flies gathered significantly at the LED end under UV light. In fact, this was almost the same as blue light and green light. In contrast, there was no significant difference as to weakgreen light, red light and infra-red light. This is consistent with previous research (e.g. 著者名 年). Furthermore, these results can be best described from the viewpoint of neuroscience. According to Yamaguchi (2010)<html><a href="http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851746/?tool=pmcentrez#r1">[3]</a></html>, the Drosophila eyes each have eight photoreceptors, R1-R8. Photoreceptors R1–R6 have the similar spectral sensitivity. Likewise, photoreceptors R7 and R8 are important for color vision. R1-R6, R7 and R8 are mainly sensitive to blue light, UV light and blue-or-green light respectively. Therefore, it can be concluded that flies are only able to perceive relatively short wavelength light such as UV light.<br/> | |
| - | </ | + | In addition, further experiments were conducted in order to demonstrate the luminous E.coli’s ability to attract flies. The results of this survey can be summarized as follows:(表を挿入) |
| + | Unfortunately, the significant difference was not observed. Perhaps this is because the intensity of the emitted light was too weak for flies to perceive the light. However, as you can see, igem 2010 Cambridge team succeeded to make E.coli emit light and to reinforce the intensity of the light. If our E.coli can emit brighter light like them, it is convincing that the researches show the significant difference. | ||
| + | (要添削) | ||
| - | + | After running t test at a significance level of 5%, it is known that the flies gathered significantly at the LED end under UV light, blue light and green light. On the other hand, when red light was used, the difference between the 2 ends was negligible. This is consistent with previously reported results. | |
| - | + | ||
| - | + | t検定を危険率5%で行った結果、紫外線、青色光、緑色光ではハエがライト点灯側に有意に多く寄ってきていたことが分かった。一方、赤色光、赤外線では有意な差は認められなかった。これは既に報告されている結果とも一致している。(←要文献) | |
| - | + | 9月26日に行った実験では、発光大腸菌による光でも有意な差を得ることはできなかった。(←要改善) | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ||
| - | + | --> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | |||
| - | + | <div style="width:100%; float:left;"> | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
== '''Reference''' == | == '''Reference''' == | ||
| - | |||
| - | [1] | + | [1] C. Hernández Salomon and H. -C. Spatz. “Colour Vision in ''Drosophila melanogaster'': Wavelength discrimination.” ''Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology'' 150 (1): pp.31-37,Spt 1982 |
| - | [2] | + | [2] Satoko Yamaguchi, et al. “Contribution of Photoreceptor Subtypes to Spectral Wavelength Preference in ''Drosophila''.” ''Proceedings of the National Academy of Sciences of the United States of America''vol 107 (12): pp.5634-5639.Feb,4, 2010 |
| - | + | </div> | |
Latest revision as of 03:49, 6 October 2011
Contents |
Project Capture
1. Introduction/Background
Methods to capture insects in the nature
In the nature, organisms capture and eat insects by various ways. For example, spiders make cobwebs to capture insects. Nepenthes has pitchers which lure and trap insects. Pyrearinus termitilluminans, which makes and lives in a tunnel around the external surface of the anthill of termite Cornitermes cumulansand, emits light in the first week of the rainy season to lure and hunt the termites.
What is the feasible method to capture insects for Carnivorous E. coli?
However, the feasible methods to capture insects for Carnivorous E. coli are limited. Because Escherichia coli moves very slowly and can only exist in the form of colonies on an agar plate or in liquid culture medium, it cannot exist in the form of complicated shapes. Considering these features of E.coli, one way for Carnivorous E. coli to capture insects is to lure the insects to itself, and bind them on the colonies or drown them in the liquid medium.
We noticed that drosophilas sometimes drown in electrophoresis tanks. Hence we are convinced that a liquid medium is a feasible and simple way to capture insects. If E.coli can lure insects(drosophilas), it can capture them.
Ways to lure insects
There are different ways to lure insects: smell, pheromone, and light.
Smell is a popular way to attract insects, as seen in some plants. For example, the Rafflesia attracts flies by it's distinctive odor like rotting flesh. Pheromone is a secreted or excreted chemical factor that triggers social responses in members of the same species. It is effective in very low concentration, and there are some pheromones which have different functions: aggregation, alarm, territory, and so on. Light is also a way to lure some kinds of insects by appealing to their phototaxis. Moths and flies have phototaxis. A few species use light to lure insects: Cornitermes cumulansand and Arachnocampa use light to hunt insects.
In this study, we focus on using light as the method to lure insects (drosophilas) because of the following reasons:
- Lighting is a visible action and we think that it is suitable for our purpose to create animal-like E. coli.
- There is an available biobrick which has the function to emit light: BBa_K3225909 created by iGEM 2010 Cambridge Team.
2. Light as a method to lure drosophilas
Drosophila melanogaster
We chose Drosophila melanogaster as one of Carnivorous E. coli’s prey, and our model organism. D. melanogaster has a positive phototaxis, lives all over Japan, and they are small (the length of its body is only a few millimeters). Besides that, it is one of the popular model organisms and available for us.
Suitable wavelength of the light to lure drosophilas
According to the article [1], drosophila melanogaster has a strong positive phototaxis to the light of wavelength shorter than 500nm.(Fig.3)
BBa_K325909, one of the biobricks created by iGEM 2010 Cambridge Team, is the lux operon from the Viblio fischeri which can be used as a L-arabinose --> blue light devise. There is a graph which shows the spectrum of light emitted by V. fischeri in 2010 Cambridge Team's WIKI(--> link). The peak wavelength is about 500 nm and we can see that the large part of light emitted by BBa_K325909 is in the range of wavelength suitable to lure drosophilas.
3. Experiment 1 --- Drosophila's phototaxis in different colors
We made an experiment about drosophila's phototaxis to the lights of different colors(ultraviolet, blue, green, red, and infrared) to show that our assay with a Y-maze is reliable.
In the Y-maze, There are two diodes at the two ends of the both sides; one is lighting and the other is not lighting.
In an assay, we gathered 5 flies with a pooter and put them into the bottom end of the Y maze. After 3 minutes, we counted the number of the flies in each side. (read the materials and methods in detail)
The results of these surveys can be summarized as follows (Vertical axis of these graphs indicates the average number of flies. error bars correspond to the standard deviation.) :
In all of these results, the number of flies in light sides is larger than those in the opposite sides. After we run the t test and chi-squared test of those data at a significance level of 5%, it was revealed that the flies gathered significantly at the LED end under UV light, blue light and green light. On the other hand, when red light was used, the difference between the 2 ends was negligible. This is consistent with the results reported by [2] that drosophilas have a strong positive phototaxis to the light of wavelength shorter than 500nm. It means that our assay of drosophila's phototaxis with Y-maze is a certain assay.
Experiment 2 --- Assay of drosophila's phototaxis to the light emitted by E.coli
We confirmed that our assay with Y-maze is reliable by experiment 1, and we intended to assay the phototaxis to the light emitted by E.coli. But unfortunately, we couldn't make strong and stable light; some of our E.coli lighted weakly and others didn't. Besides, our lighting E.coli lost its light soon.
Instead, we covered a green LED with black film and make it light as weak as our E.coli, and we assayed with the weak green LED by the Y-maze. Here is the result. Unfortunately, the significant difference was not observed. We think that it is because the intensity of the emitted light was too weak. However, as you can see, iGEM 2010 Cambridge team succeeded to make E.coli emit light and to reinforce the intensity of the light. We think that if our E.coli can emit brighter light like them, the assay will show the significant difference.
Reference
[1] C. Hernández Salomon and H. -C. Spatz. “Colour Vision in Drosophila melanogaster: Wavelength discrimination.” Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 150 (1): pp.31-37,Spt 1982
[2] Satoko Yamaguchi, et al. “Contribution of Photoreceptor Subtypes to Spectral Wavelength Preference in Drosophila.” Proceedings of the National Academy of Sciences of the United States of Americavol 107 (12): pp.5634-5639.Feb,4, 2010
 "
"