Team:Kyoto/Measurement
From 2011.igem.org
Grassfield (Talk | contribs) (→Assay of drosophila's phototaxis in different colors) |
|||
| (13 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
{{Kyoto_WikiDesign}} | {{Kyoto_WikiDesign}} | ||
| - | + | ='''Measurement'''= | |
| - | + | ==Solubilization of Antibiotics== | |
# Mix the following (Final concentration is 50mg/mL). | # Mix the following (Final concentration is 50mg/mL). | ||
#* Ampicillin: | #* Ampicillin: | ||
| Line 15: | Line 15: | ||
# Store in the -20℃ freezer. | # Store in the -20℃ freezer. | ||
| - | + | ==Transformation== | |
# Unfreeze conpitent cells on ice. | # Unfreeze conpitent cells on ice. | ||
# Dry a plate by letting the plate upside down and partly open in incubator. | # Dry a plate by letting the plate upside down and partly open in incubator. | ||
| Line 23: | Line 23: | ||
# Culture for 1h in preculture medium (LB or SOC medium), and plate by using spreader. Do not heat spreader too much because e.coli will dead for heat. | # Culture for 1h in preculture medium (LB or SOC medium), and plate by using spreader. Do not heat spreader too much because e.coli will dead for heat. | ||
| - | + | ===Sequence=== | |
# Use Big Dye Terminator 3.1(ABI) | # Use Big Dye Terminator 3.1(ABI) | ||
# Mix the following | # Mix the following | ||
| Line 35: | Line 35: | ||
# Add 25µL 100% ethanol and 1µL NAOAC | # Add 25µL 100% ethanol and 1µL NAOAC | ||
| - | + | ===Measurement of RPU=== | |
#Cultivate E.coli in M9 media(+ casamino acids) for about 15 hours. | #Cultivate E.coli in M9 media(+ casamino acids) for about 15 hours. | ||
#Dispence 2.4ml to each tube. | #Dispence 2.4ml to each tube. | ||
| Line 42: | Line 42: | ||
#Centrifuge these tubes again and discarde the supernatant and added 1.2ml media(-casamino acids) at 37 ℃. | #Centrifuge these tubes again and discarde the supernatant and added 1.2ml media(-casamino acids) at 37 ℃. | ||
#Bring up E.coli at 0,5,10,15,20,25,30,60min and extracted RNA and synthesized cDNA. | #Bring up E.coli at 0,5,10,15,20,25,30,60min and extracted RNA and synthesized cDNA. | ||
| + | |||
| + | |||
| + | ==='''preliminary experiments'''=== | ||
| + | |||
| + | '''1-1:plotting the standard line''' | ||
| + | |||
| + | |||
| + | {| | ||
| + | |Sample | ||
| + | | glucose solution(various concentration from 0.20 mM to 1.60 mM) | ||
| + | |- | ||
| + | |Blank | ||
| + | | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) | ||
| + | |} | ||
| + | |||
| + | |||
| + | #added 240㎕ DNS reagent to 80㎕sample | ||
| + | #heated it for 5 min in boiling water and then cooled it in water. | ||
| + | #added it distilled water by 2ml | ||
| + | #measured absorbance in 550nm | ||
| + | |||
| + | The standard curve is shown in fig 1. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''1-2:evaluate the affection of medium''' | ||
| + | |||
| + | we examined the interruption based on the components of media(SOC, plas-grow and M9) | ||
| + | |||
| + | |||
| + | {| | ||
| + | |Sample | ||
| + | | each medium | ||
| + | |- | ||
| + | |Blank | ||
| + | | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) | ||
| + | |} | ||
| + | |||
| + | |||
| + | #added 240㎕ DNS reagent to 80㎕sample | ||
| + | #heated it for 5 min in boiling water and then cooled it in water. | ||
| + | #added it distilled water by 2ml | ||
| + | #measured absorbance in 550nm | ||
| + | |||
| + | The result is shown in fig 2. | ||
| + | |||
| + | '''1-3:measurement the effect of remainded E.coli''' | ||
| + | |||
| + | |||
| + | {| | ||
| + | |Sample | ||
| + | | each medium | ||
| + | |- | ||
| + | |Blank | ||
| + | | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) | ||
| + | |} | ||
| + | |||
| + | |||
| + | This assay was performed three times for each medium | ||
| + | #poured the medium cultured E.coli overnight 1.2 ㎕ into each five microcentritube. | ||
| + | #centrifuged them for 5 min at 5,000 rpm | ||
| + | #prepared new five microcentritube and move 800㎕ the supernatants into each of them. | ||
| + | #measure the OD550 of one tube(use fresh medium as a blank in following assays) | ||
| + | #one hour after, we measured OD 550 of other tube | ||
| + | #take 80㎕ supernatant and move it into new tube and then heated it for 3 min in boiling water and then cooled it in water. | ||
| + | #added 240㎕ DNS reagent and heated it for 3 min in boiling water and then cooled it in water. | ||
| + | #two, three, five hours after, we did above operation, taking supernatant, measured OD500, heating and cooling, applying DNS reagent and heating and cooling again. | ||
| + | #added all sample tube (containing 320㎕ solution) distilled water by 2ml and measure the absorbance of them in 550nm. | ||
| + | |||
| + | The results of measurement OD550 are shown in fig 3 and of measurement DNS assay are shown in fig 4. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==Assay of drosophila's phototaxis with Y-maze== | ||
| + | ===1. Apparatus=== | ||
| + | ====Y maze==== | ||
| + | Y maze was constructed by a Y joint, cut blue tip, straws and LEDs.Cut blue tip had the function to prevent drosophilas to go backward. Straws and cut blue tip were fitted with the two terminals of Y joint by vinyl tape as figure.LEDs were fitted with the other terminals of the straws by 凹-form parts made of polyethylene board. The angles of Y-form was 120°. | ||
| + | |||
| + | This Y maze is put in the dark-room environment. | ||
| + | |||
| + | <div style="width:100%; float:left"> | ||
| + | [[File:Y_maze_photo.jpg|thumb|left|300px|Fig.1]] | ||
| + | |||
| + | [[File:Y maze illustration.png|thumb|left|300px|Fig.2]] | ||
| + | |||
| + | [[File:Box for Ymaze.jpg|thumb|left|250px|Fig.3 : Our dark-room environment]] | ||
| + | </div> | ||
| + | |||
| + | Here is the list of the LEDs which we used in the experiment. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ The list of our LEDs | ||
| + | ! Color !! Manufacturer !! No. !! Name !! Wavelength | ||
| + | |- | ||
| + | ! Ultraviolet|| OptoSupply || OSSV5111A || 5mm Super Violet LED || Peak wavelength = 400 nm | ||
| + | |- | ||
| + | ! Blue|| OptoSupply || OSUB5161P || 5mm Deluxe Blue LED || Dominant wavelength = 465~475 nm | ||
| + | |- | ||
| + | ! Green|| SHARP || GL3GC402B0SC || High-luminosity(InGaN) LED Lamp || Dominant wavelength = 525 nm | ||
| + | |- | ||
| + | ! Red|| OptoSupply || OSHR5161P || 5mm Deluxe Red LED || Dominant wavelength = 620~630 nm | ||
| + | |- | ||
| + | ! Infrared || OptoSupply || OSIR5113A || 5mm Infrared Emitting Diode || Peak wavelength = 940 nm | ||
| + | |} | ||
| + | |||
| + | ===Pooter=== | ||
| + | A pooter is very useful tool to move drosophilas. | ||
| + | We also used it to move them from rearing bottles to the Y maze. | ||
| + | <br><br> | ||
| + | Our pooter consisted of two parts: tube part and tip part. | ||
| + | The tube part was made of vinyl tube and two cutted tips of pipetman. | ||
| + | The cutted tips were fitted with the terminals of tube by vinyl tape as mouthpiece and objective end. | ||
| + | The tip part was made of two cutted tips of pipetman and a piece of tissue paper. | ||
| + | Not to inhale drosophilas into the mouth, the piece of tissue paper was slipped in between the cutted tips. | ||
| + | <br><br> | ||
| + | When we move drosophilasfrom rearing bottles to the Y maze, we fitted the tip part with the tube part and draw some drosophilas into the tip part by mouth. Then closing the orifice of tip part by finger, we removed the tip part from the tube part and put it into the Y maze. | ||
| + | |||
| + | <div style="width:100%; float:left"> | ||
| + | [[File:Pooter photo.jpg|thumb|left|300px|Fig.3]] | ||
| + | |||
| + | [[File:Pooter illustration.png|thumb|left|300px|Fig.4]] | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <!-- | ||
| + | Straw was constructed by the commonly available straw, plastic tip and tissue. The tips were used as mouthpiece and objective end. | ||
| + | |||
| + | [[File:吸虫管.jpg]] | ||
| + | |||
| + | ここに装置全体の写真とイラスト(完成図と制作方法)を載せる。 | ||
| + | |||
| + | ↑なし/ Diagrammatic representation of the pooter constructed from a straw, pipets and tissue. | ||
| + | --> | ||
| + | |||
| + | ==2. Drosophilas== | ||
| + | We used the Oregon-RS of Drosophila melanogaster. They were derived from Dr. Fuse's laboratory on 4 August 2011. They were maintained in an incubator at 22℃ on standard cornmeal medium in the ordinary glass bottle <!--(____cm high, ____cm in diameter)--> with a 12h light phase (12:12 light:dark cycle). Flies were aged 2-4 days after emergence. | ||
| + | |||
| + | ==3. Method== | ||
| + | Firstly, we gathered 5 flies with a pooter. After that, we removed the tip of the pooter and attached the tip to the bottom end of the Y maze. Then we left the flies intact in the dark for three minutes and counted the number of flies in each tube. In this experiment, we switched both the left and right LEDs on for 2 times alternatively. We ran the experiment 4 times using 2 sets of 5 male flies and 2 sets of 5 female flies.<!--We summed up the number of flies that entered the lighting LED end and opposite end and ran the t test.--> | ||
| + | |||
| + | [[File:Ymaze experiment method.PNG|thumb|center|600px]] | ||
| + | |||
| + | <!-- | ||
| + | ハエ5匹を吸虫管で取る。吸虫管チップ部を取り外し、Y mazeの下部に差し込み、取り付ける。暗黒下で3分間放置し、Y mazeのどちらの管に何匹入ったかをカウントした。実験は左のライトを点灯した場合を2回、右のライトを点灯した場合を2回測定した。オス5匹1セットを2組、メス5匹1セットを2組行い、ライト点灯側と反対側に入ったハエの数のそれぞれ合計をとり、t検定を行った。(by 草場) | ||
| + | --> | ||
Latest revision as of 03:34, 6 October 2011
Contents |
Measurement
Solubilization of Antibiotics
- Mix the following (Final concentration is 50mg/mL).
- Ampicillin:
- Ampicillin
- 1.0g
- MilliQ
- 20mL
- Kanamycin:
- Kanamycin
- 0.5g
- MilliQ
- 10mL
- Ampicillin:
- Dispense 1.1mL of the solution into 1.5mL tubes.
- Store in the -20℃ freezer.
Transformation
- Unfreeze conpitent cells on ice.
- Dry a plate by letting the plate upside down and partly open in incubator.
- Add 1µL DNA solution and 20µL compitent cells to 1.5mL tube, let stand for 30min on ice. If few colony is observed, increase the amount of the compitent cells or DNA, but make the amount of DNA not to get over that of the compitent cells.
- Heatshock for 60s at 42℃.
- Let stand for 2min on ice.
- Culture for 1h in preculture medium (LB or SOC medium), and plate by using spreader. Do not heat spreader too much because e.coli will dead for heat.
Sequence
- Use Big Dye Terminator 3.1(ABI)
- Mix the following
- 5xBuffer
- 2µL
- Primer (3.2µM)
- 1µL
- Template Plasmid
- 200ng
- Big Dye Terminator 3.1
- 0.5µL
- MilliQ
- up to 10µL
- Let stand for 1min at 96℃.
- 35 cycles for 5s at 98℃, for 5s 50℃, and for 2.5min at 68℃.
- Add 25µL 100% ethanol and 1µL NAOAC
Measurement of RPU
- Cultivate E.coli in M9 media(+ casamino acids) for about 15 hours.
- Dispence 2.4ml to each tube.
- Centrifuge these tubes (13,000 rpm , 4℃, 1min) and discarded the supernatant.
- Add 1.2ml media(- casamino acids) and centrifuged at 4℃ twice.
- Centrifuge these tubes again and discarde the supernatant and added 1.2ml media(-casamino acids) at 37 ℃.
- Bring up E.coli at 0,5,10,15,20,25,30,60min and extracted RNA and synthesized cDNA.
preliminary experiments
1-1:plotting the standard line
| Sample | glucose solution(various concentration from 0.20 mM to 1.60 mM) |
| Blank | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) |
- added 240㎕ DNS reagent to 80㎕sample
- heated it for 5 min in boiling water and then cooled it in water.
- added it distilled water by 2ml
- measured absorbance in 550nm
The standard curve is shown in fig 1.
1-2:evaluate the affection of medium
we examined the interruption based on the components of media(SOC, plas-grow and M9)
| Sample | each medium |
| Blank | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) |
- added 240㎕ DNS reagent to 80㎕sample
- heated it for 5 min in boiling water and then cooled it in water.
- added it distilled water by 2ml
- measured absorbance in 550nm
The result is shown in fig 2.
1-3:measurement the effect of remainded E.coli
| Sample | each medium |
| Blank | mixed liquid (240μℓDNS reagent plus 1760㎕distilled water ) |
This assay was performed three times for each medium
- poured the medium cultured E.coli overnight 1.2 ㎕ into each five microcentritube.
- centrifuged them for 5 min at 5,000 rpm
- prepared new five microcentritube and move 800㎕ the supernatants into each of them.
- measure the OD550 of one tube(use fresh medium as a blank in following assays)
- one hour after, we measured OD 550 of other tube
- take 80㎕ supernatant and move it into new tube and then heated it for 3 min in boiling water and then cooled it in water.
- added 240㎕ DNS reagent and heated it for 3 min in boiling water and then cooled it in water.
- two, three, five hours after, we did above operation, taking supernatant, measured OD500, heating and cooling, applying DNS reagent and heating and cooling again.
- added all sample tube (containing 320㎕ solution) distilled water by 2ml and measure the absorbance of them in 550nm.
The results of measurement OD550 are shown in fig 3 and of measurement DNS assay are shown in fig 4.
Assay of drosophila's phototaxis with Y-maze
1. Apparatus
Y maze
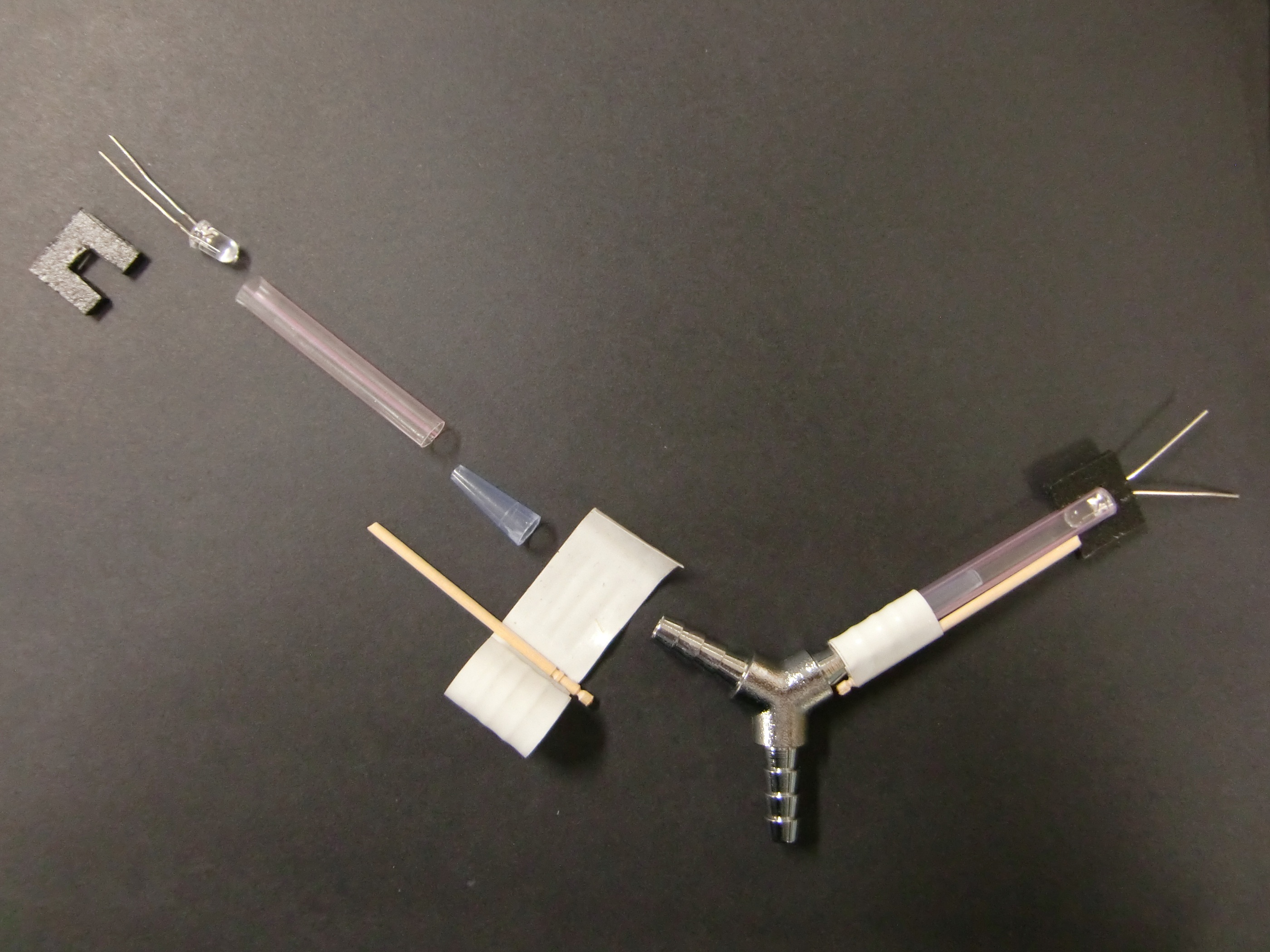
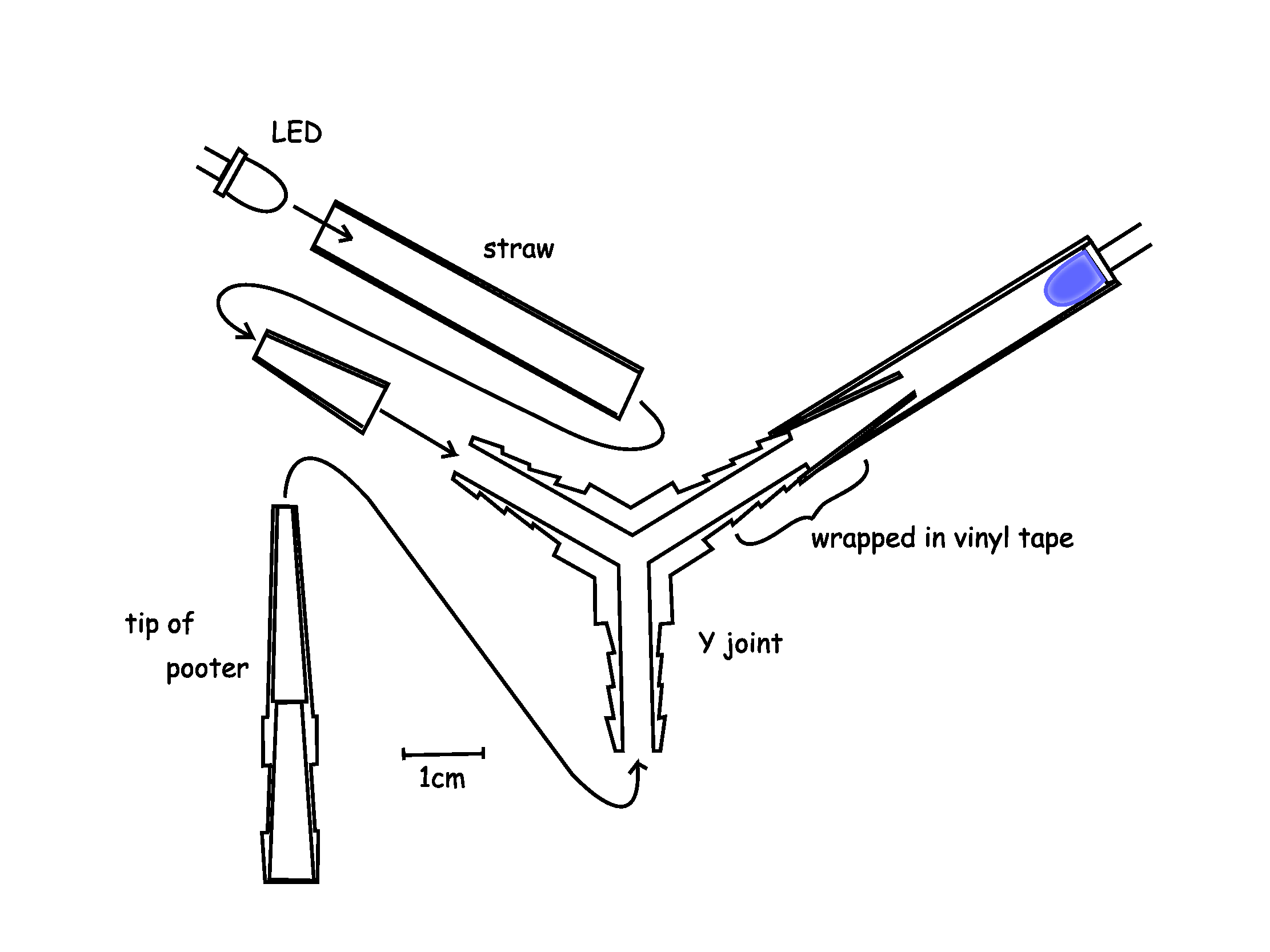
Y maze was constructed by a Y joint, cut blue tip, straws and LEDs.Cut blue tip had the function to prevent drosophilas to go backward. Straws and cut blue tip were fitted with the two terminals of Y joint by vinyl tape as figure.LEDs were fitted with the other terminals of the straws by 凹-form parts made of polyethylene board. The angles of Y-form was 120°.
This Y maze is put in the dark-room environment.
Here is the list of the LEDs which we used in the experiment.
| Color | Manufacturer | No. | Name | Wavelength |
|---|---|---|---|---|
| Ultraviolet | OptoSupply | OSSV5111A | 5mm Super Violet LED | Peak wavelength = 400 nm |
| Blue | OptoSupply | OSUB5161P | 5mm Deluxe Blue LED | Dominant wavelength = 465~475 nm |
| Green | SHARP | GL3GC402B0SC | High-luminosity(InGaN) LED Lamp | Dominant wavelength = 525 nm |
| Red | OptoSupply | OSHR5161P | 5mm Deluxe Red LED | Dominant wavelength = 620~630 nm |
| Infrared | OptoSupply | OSIR5113A | 5mm Infrared Emitting Diode | Peak wavelength = 940 nm |
Pooter
A pooter is very useful tool to move drosophilas.
We also used it to move them from rearing bottles to the Y maze.
Our pooter consisted of two parts: tube part and tip part.
The tube part was made of vinyl tube and two cutted tips of pipetman.
The cutted tips were fitted with the terminals of tube by vinyl tape as mouthpiece and objective end.
The tip part was made of two cutted tips of pipetman and a piece of tissue paper.
Not to inhale drosophilas into the mouth, the piece of tissue paper was slipped in between the cutted tips.
When we move drosophilasfrom rearing bottles to the Y maze, we fitted the tip part with the tube part and draw some drosophilas into the tip part by mouth. Then closing the orifice of tip part by finger, we removed the tip part from the tube part and put it into the Y maze.
2. Drosophilas
We used the Oregon-RS of Drosophila melanogaster. They were derived from Dr. Fuse's laboratory on 4 August 2011. They were maintained in an incubator at 22℃ on standard cornmeal medium in the ordinary glass bottle with a 12h light phase (12:12 light:dark cycle). Flies were aged 2-4 days after emergence.
3. Method
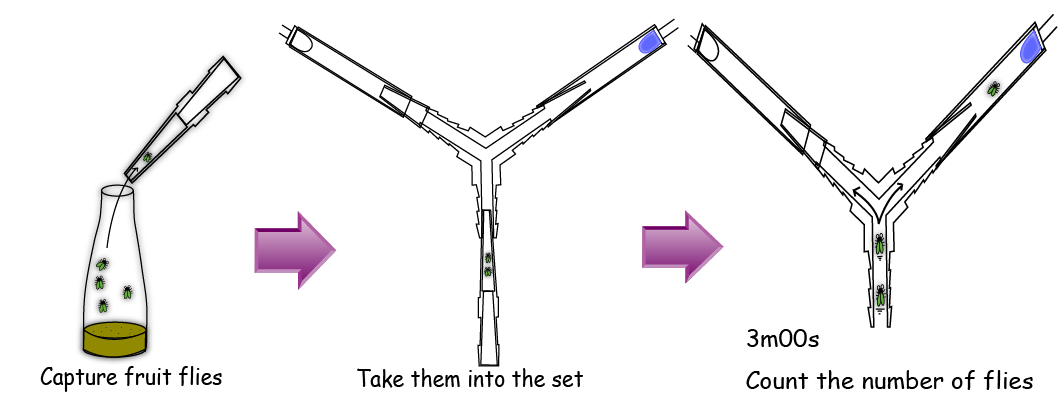
Firstly, we gathered 5 flies with a pooter. After that, we removed the tip of the pooter and attached the tip to the bottom end of the Y maze. Then we left the flies intact in the dark for three minutes and counted the number of flies in each tube. In this experiment, we switched both the left and right LEDs on for 2 times alternatively. We ran the experiment 4 times using 2 sets of 5 male flies and 2 sets of 5 female flies.
 "
"