Team:HIT-Harbin/Project
From 2011.igem.org
(→Conclusion) |
|||
| (32 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:HIT-Harbin/header}} | {{:Team:HIT-Harbin/header}} | ||
| - | |||
| - | |||
__TOC__ | __TOC__ | ||
| - | + | ='''Overview'''= | |
| - | + | <div class="cadre"><br/>Since lots of people in China are lactose-intolerant, they have no access to drinking milk in the past. With the development of the dairy industry in China, yogurt has become highly accepted by consumers, including those lactose-intolerant people. And postacidification has always been the most vital factor which affects the shelf life and flavor of yogurt. So our team takes it as our track. Through the literature, we have discovered a gene, called ''lacR'', which could combine with the lactose operon to inhibit the production of lactic acid in ''Bulgaria Lactobacillus''. If ''lacR'' could be highly transcripted in Bulgaria Lactobacillus when the pH value of yogurt declines to 5.5 or lower, the acidification of yogurt would be minimized. Meanwhile, we also want to transfer part of human collagen genes to ''Streptococcus Thermophilus'' in order to enhance the nutrition of yogurt.<br/><br/></div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | + | ='''Background'''= | |
| - | < | + | =='''Why yogurt'''== |
| + | <div class="cadre"><br/>Yogurt is manufactured using a culture of ''Lactobacillus delbrueckii'' subsp. ''bulgaricus'' and ''Streptococcus salivarius'' subsp. ''thermophilus'' bacteria. This fermented product is nutritionally rich in protein, calcium, riboflavin, vitamin B<sub>6</sub> and vitamin B<sub>12</sub><sup>[1]</sup>. In addition, consumers who are moderately lactose-intolerant can consume yoghurt without ill symptoms, because much of the lactose in the milk precursor is converted to lactic acid by the bacterial culture<sup>[2]</sup>. That is very important to Chinese people for many of them are lactose-intolerant (FIG 2.1). Besides, yoghurt containing live cultures is sometimes used in an attempt to prevent antibiotic-associated diarrhea. Yoghurt contains varying amounts of fat. There is non-fat (<0.5% fat), low-fat (usually 2% fat) and plain or whole milk yoghurt (4% fat). A study published in the International Journal of Obesity also found that the consumption of low-fat yoghurt can promote weight loss, mainly due to the abundance of calcium in the yogurt<sup>[3]</sup>.<br/> | ||
| + | <center><br/>[[File:Msybus.jpg]]<br/></center> | ||
<br/> | <br/> | ||
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | =='''About post-acidification'''== | |
| - | + | <div class="cadre"><br/>Although yogurt possesses many health benefits there exists a major problem in yogurt manufacturing and during storage prior to consumption, i.e., post-acidification. As bioactive ingredients, ''Lactobacillus bulgaricus'' and ''Streptococcus thermophilus'' continue to produce lactic acid after production fermentation, making the yogurt too sour. This phenomenon is not desirable. Post-acidification shortens yogurt’s shelf life results in an unacceptable taste by consumers. To control the postacidification of yogurts, the supply chain which is called cold chain has been widely used. A cold chain, especially an unbroken cold chain, is an uninterrupted series of storage and distribution activities which maintain a given temperature range. However, it could be too difficult to reach the rules in developing countries. For most of the time, the yogurts in these countries are stored in the room temperature or under a cold condition which is not low enough. Amount of ways have been tried to control the postacidification of yogurts when stored in higher temperature. But the effects are limited. Therefore, the objective of this project is to minimize post-acidification in yogurt achieving a consistent acidity (pH) and a prolonged shelf-life using the method of synthetic biology.<br/><br/> | |
| - | + | </div> | |
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | =='''About collagen'''== | ||
| + | <div class="cadre"><br/>Collagen is well known to consumers, especially to female consumers. Collagen is one of the long, fibrous structural proteins, whose functions are quite different from those of globular proteins such as enzymes. Tough bundles of collagen called collagen fibers are a major component of the extracellular matrix that supports most tissues and gives cells structure from the outside. Collagen is also found inside certain cells. Collagen has great tensile strength and is the main component of fascia, cartilage, ligaments, tendons, bone and skin<sup>[4]</sup>. Along with soft keratin, it is responsible for skin strength and elasticity, and its degradation leads to wrinkles with aging. It strengthens blood vessels and plays an important role in tissue development. It is present in the cornea and lens of the eye in crystalline form. Hydrolyzed collagen can also play an important role in weight management, as a protein, it can be advantageously used for its satiating power. Thus it is of great interest in producing a yogurt rich in collagen.<br/><br/> | ||
| + | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ='''Outline'''= | |
| + | <div class="cadre"><br/>In order to achieve our goals, we have designed four new biobricks. With these biobricks, three steps are needed to minimize the process of post-acidification and produce collagen in yogurt. First, a food-grade vector is being designed. Secondly, biobricks for minimizing postacidification are being reconstructed with a food-grade vector and the recombinant plasmid will be transduced into ''Lactobacillus bulgaricus''. Thirdly, the food-grade vector reconstructed with the genes coding a section of human collagen will be transduced into ''Streptococcus thermophilus''.<br/><br/> | ||
| + | </div> | ||
| - | |||
| + | =='''Food-grade vector'''== | ||
| + | <div class="cadre"><br/>The proposed food-grade vector is based on the plasmid pMG36e, which has the erythromycin resistance gene from Staphylococcus aureuis. The plasmid, pMG36e, functions in a wide range of bacteria <sup>[5]</sup>. These include ''E. coli'', ''B. subtilis'' and especially ''Lactococcus lactis''. But for a food-grade vector, we need to change the antibiotic gene to a safer resistance gene to avoid potential risks. A Nisin resistance gene ''nisI'' becomes our interest. Nisin is an antimicrobial peptide produced by ''Lactococcus lactis''. It has a long history of safe use in food production <sup>[6]</sup>. So we insert the ''nisI'' gene between XbaI and PstI. And then, we cut off the erythromycin resistance gene using primers with EcoRI in both ends <sup>[7]</sup> (FIG 3.1). For a better function, the coding of nisI gene has being optimized separately in ''L. bulgaricus'' and ''S. thermophilus''. | ||
| + | <center><br/>[[File:The_process_of_reconstrution.jpg]]<br/></center> | ||
| + | <br/> | ||
| + | </div> | ||
| - | + | =='''Devices for minimizing the process of postacidification'''== | |
| - | + | <div class="cadre"><br/>Yogurt will become more and more acid for the two strains, ''L. bulgaricus'' and ''S. thermophilus'', keep producing lactic acid all the time, especially ''L. bulgaricus''. To reduce the production of lactic acid, ''L. bulgaricus'' needs to perform two functions. First, it should have a pH sensor to sense the external pH value and release a signal when the pH value declines to 5.5 or lower. Then, with the signal, ''L. bulgaricus'' should cut off the pathway for lactic acid.<br/><br/> | |
| + | </div> | ||
| - | ===''' | + | ==='''pH sensor'''=== |
| - | + | <div class="cadre"><br/>Although ''L. bulgaricus'' is one of the most extensively studied lactic acid bacteria, little information is available on the acid induced gene expression. The ''rcfB'' promoter is the most recently reported gene which is highly induced by acidity. When the external pH value declines to 5.5, ''rcfB'' promoter will be highly upregulated <sup>[8]</sup>. Although, the ''rcfB'' gene, encoding RcfB protein which has unknown function, is found in ''Lactococcus lactis IL1403'', we still try to figure out whether it will work in ''L. bulgaricus''.<br/><br/> | |
| + | </div> | ||
| + | ==='''Repressor'''=== | ||
| + | <div class="cadre"><br/>Two important enzymes are needed for the lactic acid bacteria to convert lactose to glucose and then lactic acid. They are lactose permease and β-galactosidase which are located in the lac operon (FIG 3.2). In ''L. delbrueckii'' subsp. ''lactis'', the lactose permease (''lacS ''), the β-galactosidase (''lacZ '') and the repressor (''lacR '') constitute the lac operon per se. The three genes are linked together as a ''lacSZR'' operon without any promoter in between. The repressor is able to bind to the promoter region which is located upstream the ''lacS'' gene. Thus the transport of the lactose is being blocked. However, in ''L. delbrueckii'' subsp. ''bulgaricus'', the ''lacR'' gene was inactivated by small nucleotides insertions and deletions in the sequence resulting in the constitutive phenotype of this subspecies <sup>[9]</sup>(FIG 3.3). So, the normal express of ''lacR'' would cut off the pathway for lactic acid production. The overview of the pH sensor and repressor is shown in FIG 3.4. | ||
| + | <center><br/>[[File:The_metabolic_pathway_of_lactose.jpg]]<br/></center> | ||
| + | <center><br/>[[File:The_lac_operon_in_L.bulgaricus.jpg]]<br/></center> | ||
| + | <center><br/>[[File:Sensor_and_repressor.jpg]]<br/></center> | ||
| + | <br/> | ||
| + | </div> | ||
| - | ==''' | + | =='''Diagram for producing collagen'''== |
| + | <div class="cadre"><br/>Our collagen gene contains a coding region encoding 101 amino acids, which comes from the α<sub>1</sub> chain of human collagen type Ⅲ (''COLⅢ''), a signal peptide called ''blpC'' and a His tag (FIG 3.5). The gene ''blpC'', which is found in ''Streptococcus thermophilus CNRZ1066'', will transport the collagen out of the bacteria <sup>[10]</sup>. And the His tag is for the purification of the collagen. | ||
| + | <center><br/>[[File:The_parts_for_producing_collagen.jpg]]<br/></center> | ||
| + | <br/> | ||
| + | </div> | ||
| - | <br/> | + | ='''Conclusion'''= |
| + | <div class="cadre"><br/>1. The parts for minimizing the process of postacidification and producing of collagen have been ligated with pMG36e (for the convenience of screening in ''E.coli'' and the erythromycin resistance gene will be knocked off later). The figures below showed the recombinant plasmids(digested with Xbal and Pstl) in 1.0% agarose gel electrophoresis.<br/> | ||
| + | <center>[[File:The_recombinant_plasmid_for_minimizing_the_process_of_postacidification.jpg]] [[File:The_restriction_pattern_of_recombinant_plasmid_for_producing_collagen.jpg]]</center> | ||
| + | <br/>2. The recombinant plasmids have been transduced into ''L.burlgaricus'' and ''S.thermophilus'' separately by electricity transduction. Further experiments are under way.<br/><br/> | ||
| - | |||
| - | |||
| + | </div> | ||
| - | + | ='''References'''= | |
| - | + | <div class="cadre"> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | = | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br/>[1] J C Kolars, M D Levitt, M Aouji, ''et al''. (1984). Yogurt-An Autodigesting Source of Lactose [J]. New England Journal of Medicine, 310: 1-3.<br/> | <br/>[1] J C Kolars, M D Levitt, M Aouji, ''et al''. (1984). Yogurt-An Autodigesting Source of Lactose [J]. New England Journal of Medicine, 310: 1-3.<br/> | ||
| - | + | [2] R S Beniwal, V C Arena, L Thomas, ''et al''. (2003). A Randomized Trial of Yogurt for Preverntion of Antibiotic-Associated Diarrhea [J]. Digestive Disease and Sciences, 48(10): 2077-2082.<br/> | |
| - | [2] R S Beniwal, V C Arena, L Thomas, ''et al''. (2003). A Randomized Trial of Yogurt for Preverntion of Antibiotic-Associated Diarrhea [J]. Digestive Disease and Sciences, 48(10): 2077-2082. | + | [3] M B Zemel, J Richards, S Mathis, ''et al''. (2005). Dairy augmentation of total and central fat loss in obese subjects [J]. International Journal of Obesity, 29: 391-397.<br/> |
| - | + | [4] M J Buehler. (2006). Nature Designs Tough Collagen: Explaining the Nanostructure of Collagen Fibrils [J]. Applied Physical Sciences, Applied Biological Sciences, 103(33): 12285-12290.<br/> | |
| - | [3] M B Zemel, J Richards, S Mathis, ''et al''. (2005). Dairy augmentation of total and central fat loss in obese subjects [J]. International Journal of Obesity, 29: 391-397. | + | [5] Maarten Van De Guchte, Jos M. B. M. Van Der Vossen, Jan Kok, ''et al''. (1989). Construction of a Lactococcal Expression Vector: Expression of Hen Egg White Lysozyme in Lactococcus lactis subsp. lactis [J]. Applied and Environmental Microbiology, 55(1): 224-228.<br/> |
| - | + | [6] Aljoša Trmčić, John Samelis, Christophe Monnet, ''et al''. (2011). Complete nisin A gene cluster from Lactococcus lactis M78 (HM219853) – obtaining the nucleic acid sequence and comparing it to other published nisin sequences [J]. Genes and Genomics, 33: 217-221.<br/> | |
| - | [4] M J Buehler. (2006). Nature Designs Tough Collagen: Explaining the Nanostructure of Collagen Fibrils [J]. Applied Physical Sciences, Applied Biological Sciences, 103(33): 12285-12290. | + | [7] Wang Cheng. (2010). Construction of an expression vector with Nisin resistant gene nisI as a food grade selection marker for Lactococcus lactis [D]. South China University of Technology.<br/> |
| - | + | [8] Ismail Akyol, Ugur Coplekcioglu, Asuman Karakas, ''et al''. (2008). Regulation of the acid inducible rcfB promoter in Lactococcus lactis subsp. lactis. Annals of Microbiology, 58(2): 269-273.<br/> | |
| - | [5] Maarten Van De Guchte, Jos M. B. M. Van Der Vossen, Jan Kok, ''et al''. (1989). Construction of a Lactococcal Expression Vector: Expression of Hen Egg White Lysozyme in Lactococcus lactis subsp. lactis [J]. Applied and Environmental Microbiology, 55(1): 224-228. | + | [9] Jacques Edouard Germond, Luciane LaPierre, Beat Mollet, ''et al''. Expression contructs using Lactobacillus delbrueckii subsp. lactis lac repressor protein and its lac repressor binding site, miroorganisms and methods thereof (P). United States Patent: US 6929931 B1. Aug. 16, 2005.<br/> |
| - | + | [10] Bolotin A, ''et al''. (2004). Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus [J]. Nat Biotechnol, 22(12):1554-1558.<br/> | |
| - | [6] Aljoša Trmčić, John Samelis, Christophe Monnet, ''et al''. (2011). Complete nisin A gene cluster from Lactococcus lactis M78 (HM219853) – obtaining the nucleic acid sequence and comparing it to other published nisin sequences [J]. Genes and Genomics, 33: 217-221. | + | </div> |
| - | + | ||
| - | [7] Wang Cheng. (2010). Construction of an expression vector with Nisin resistant gene nisI as a food grade selection marker for Lactococcus lactis [D]. South China University of Technology. | + | |
| - | + | ||
| - | [8] Ismail Akyol, Ugur Coplekcioglu, Asuman Karakas, ''et al''. (2008). Regulation of the acid inducible rcfB promoter in Lactococcus lactis subsp. lactis. Annals of Microbiology, 58(2): 269-273. | + | |
| - | + | ||
| - | [9] Jacques Edouard Germond, Luciane LaPierre, Beat Mollet, ''et al''. Expression contructs using Lactobacillus delbrueckii subsp. lactis lac repressor protein and its lac repressor binding site, miroorganisms and methods thereof (P). United States Patent: US 6929931 B1. Aug. 16, 2005. | + | |
| - | + | ||
| - | [10] Bolotin A, ''et al''. (2004). Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus [J]. Nat Biotechnol, 22(12):1554- | + | |
| - | + | ||
| - | + | ||
{{:Team:HIT-Harbin/footer}} | {{:Team:HIT-Harbin/footer}} | ||
Latest revision as of 15:29, 5 October 2011
Contents |
Overview
Since lots of people in China are lactose-intolerant, they have no access to drinking milk in the past. With the development of the dairy industry in China, yogurt has become highly accepted by consumers, including those lactose-intolerant people. And postacidification has always been the most vital factor which affects the shelf life and flavor of yogurt. So our team takes it as our track. Through the literature, we have discovered a gene, called lacR, which could combine with the lactose operon to inhibit the production of lactic acid in Bulgaria Lactobacillus. If lacR could be highly transcripted in Bulgaria Lactobacillus when the pH value of yogurt declines to 5.5 or lower, the acidification of yogurt would be minimized. Meanwhile, we also want to transfer part of human collagen genes to Streptococcus Thermophilus in order to enhance the nutrition of yogurt.
Background
Why yogurt
Yogurt is manufactured using a culture of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus bacteria. This fermented product is nutritionally rich in protein, calcium, riboflavin, vitamin B6 and vitamin B12[1]. In addition, consumers who are moderately lactose-intolerant can consume yoghurt without ill symptoms, because much of the lactose in the milk precursor is converted to lactic acid by the bacterial culture[2]. That is very important to Chinese people for many of them are lactose-intolerant (FIG 2.1). Besides, yoghurt containing live cultures is sometimes used in an attempt to prevent antibiotic-associated diarrhea. Yoghurt contains varying amounts of fat. There is non-fat (<0.5% fat), low-fat (usually 2% fat) and plain or whole milk yoghurt (4% fat). A study published in the International Journal of Obesity also found that the consumption of low-fat yoghurt can promote weight loss, mainly due to the abundance of calcium in the yogurt[3].

About post-acidification
Although yogurt possesses many health benefits there exists a major problem in yogurt manufacturing and during storage prior to consumption, i.e., post-acidification. As bioactive ingredients, Lactobacillus bulgaricus and Streptococcus thermophilus continue to produce lactic acid after production fermentation, making the yogurt too sour. This phenomenon is not desirable. Post-acidification shortens yogurt’s shelf life results in an unacceptable taste by consumers. To control the postacidification of yogurts, the supply chain which is called cold chain has been widely used. A cold chain, especially an unbroken cold chain, is an uninterrupted series of storage and distribution activities which maintain a given temperature range. However, it could be too difficult to reach the rules in developing countries. For most of the time, the yogurts in these countries are stored in the room temperature or under a cold condition which is not low enough. Amount of ways have been tried to control the postacidification of yogurts when stored in higher temperature. But the effects are limited. Therefore, the objective of this project is to minimize post-acidification in yogurt achieving a consistent acidity (pH) and a prolonged shelf-life using the method of synthetic biology.
About collagen
Collagen is well known to consumers, especially to female consumers. Collagen is one of the long, fibrous structural proteins, whose functions are quite different from those of globular proteins such as enzymes. Tough bundles of collagen called collagen fibers are a major component of the extracellular matrix that supports most tissues and gives cells structure from the outside. Collagen is also found inside certain cells. Collagen has great tensile strength and is the main component of fascia, cartilage, ligaments, tendons, bone and skin[4]. Along with soft keratin, it is responsible for skin strength and elasticity, and its degradation leads to wrinkles with aging. It strengthens blood vessels and plays an important role in tissue development. It is present in the cornea and lens of the eye in crystalline form. Hydrolyzed collagen can also play an important role in weight management, as a protein, it can be advantageously used for its satiating power. Thus it is of great interest in producing a yogurt rich in collagen.
Outline
In order to achieve our goals, we have designed four new biobricks. With these biobricks, three steps are needed to minimize the process of post-acidification and produce collagen in yogurt. First, a food-grade vector is being designed. Secondly, biobricks for minimizing postacidification are being reconstructed with a food-grade vector and the recombinant plasmid will be transduced into Lactobacillus bulgaricus. Thirdly, the food-grade vector reconstructed with the genes coding a section of human collagen will be transduced into Streptococcus thermophilus.
Food-grade vector
The proposed food-grade vector is based on the plasmid pMG36e, which has the erythromycin resistance gene from Staphylococcus aureuis. The plasmid, pMG36e, functions in a wide range of bacteria [5]. These include E. coli, B. subtilis and especially Lactococcus lactis. But for a food-grade vector, we need to change the antibiotic gene to a safer resistance gene to avoid potential risks. A Nisin resistance gene nisI becomes our interest. Nisin is an antimicrobial peptide produced by Lactococcus lactis. It has a long history of safe use in food production [6]. So we insert the nisI gene between XbaI and PstI. And then, we cut off the erythromycin resistance gene using primers with EcoRI in both ends [7] (FIG 3.1). For a better function, the coding of nisI gene has being optimized separately in L. bulgaricus and S. thermophilus.

Devices for minimizing the process of postacidification
Yogurt will become more and more acid for the two strains, L. bulgaricus and S. thermophilus, keep producing lactic acid all the time, especially L. bulgaricus. To reduce the production of lactic acid, L. bulgaricus needs to perform two functions. First, it should have a pH sensor to sense the external pH value and release a signal when the pH value declines to 5.5 or lower. Then, with the signal, L. bulgaricus should cut off the pathway for lactic acid.
pH sensor
Although L. bulgaricus is one of the most extensively studied lactic acid bacteria, little information is available on the acid induced gene expression. The rcfB promoter is the most recently reported gene which is highly induced by acidity. When the external pH value declines to 5.5, rcfB promoter will be highly upregulated [8]. Although, the rcfB gene, encoding RcfB protein which has unknown function, is found in Lactococcus lactis IL1403, we still try to figure out whether it will work in L. bulgaricus.
Repressor
Two important enzymes are needed for the lactic acid bacteria to convert lactose to glucose and then lactic acid. They are lactose permease and β-galactosidase which are located in the lac operon (FIG 3.2). In L. delbrueckii subsp. lactis, the lactose permease (lacS ), the β-galactosidase (lacZ ) and the repressor (lacR ) constitute the lac operon per se. The three genes are linked together as a lacSZR operon without any promoter in between. The repressor is able to bind to the promoter region which is located upstream the lacS gene. Thus the transport of the lactose is being blocked. However, in L. delbrueckii subsp. bulgaricus, the lacR gene was inactivated by small nucleotides insertions and deletions in the sequence resulting in the constitutive phenotype of this subspecies [9](FIG 3.3). So, the normal express of lacR would cut off the pathway for lactic acid production. The overview of the pH sensor and repressor is shown in FIG 3.4.

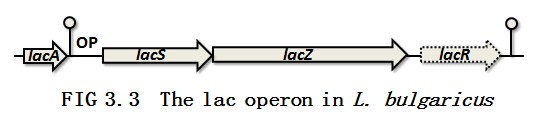
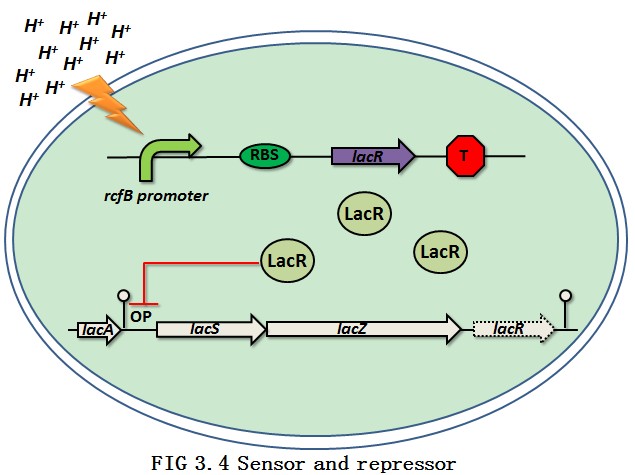
Diagram for producing collagen
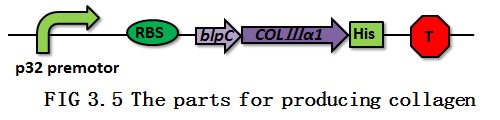
Our collagen gene contains a coding region encoding 101 amino acids, which comes from the α1 chain of human collagen type Ⅲ (COLⅢ), a signal peptide called blpC and a His tag (FIG 3.5). The gene blpC, which is found in Streptococcus thermophilus CNRZ1066, will transport the collagen out of the bacteria [10]. And the His tag is for the purification of the collagen.
Conclusion
1. The parts for minimizing the process of postacidification and producing of collagen have been ligated with pMG36e (for the convenience of screening in E.coli and the erythromycin resistance gene will be knocked off later). The figures below showed the recombinant plasmids(digested with Xbal and Pstl) in 1.0% agarose gel electrophoresis.
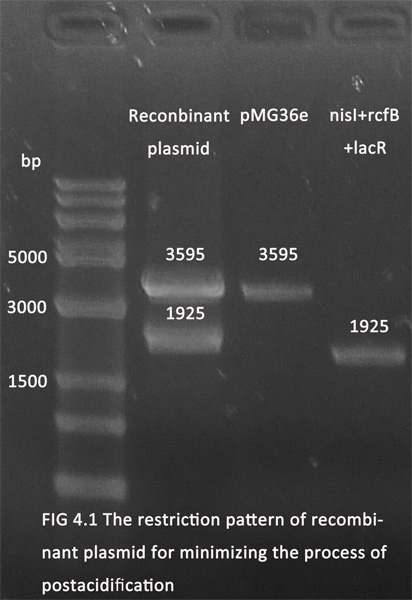
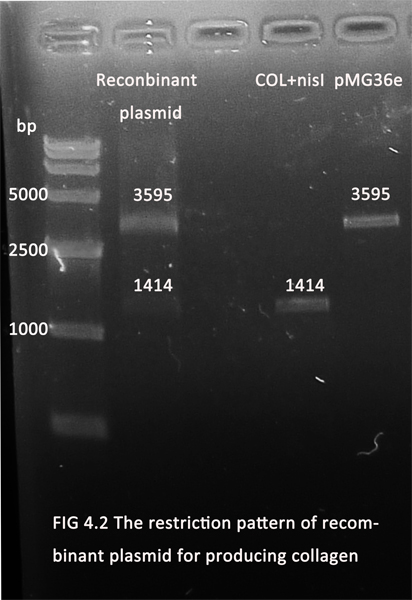
2. The recombinant plasmids have been transduced into L.burlgaricus and S.thermophilus separately by electricity transduction. Further experiments are under way.
References
[1] J C Kolars, M D Levitt, M Aouji, et al. (1984). Yogurt-An Autodigesting Source of Lactose [J]. New England Journal of Medicine, 310: 1-3.
[2] R S Beniwal, V C Arena, L Thomas, et al. (2003). A Randomized Trial of Yogurt for Preverntion of Antibiotic-Associated Diarrhea [J]. Digestive Disease and Sciences, 48(10): 2077-2082.
[3] M B Zemel, J Richards, S Mathis, et al. (2005). Dairy augmentation of total and central fat loss in obese subjects [J]. International Journal of Obesity, 29: 391-397.
[4] M J Buehler. (2006). Nature Designs Tough Collagen: Explaining the Nanostructure of Collagen Fibrils [J]. Applied Physical Sciences, Applied Biological Sciences, 103(33): 12285-12290.
[5] Maarten Van De Guchte, Jos M. B. M. Van Der Vossen, Jan Kok, et al. (1989). Construction of a Lactococcal Expression Vector: Expression of Hen Egg White Lysozyme in Lactococcus lactis subsp. lactis [J]. Applied and Environmental Microbiology, 55(1): 224-228.
[6] Aljoša Trmčić, John Samelis, Christophe Monnet, et al. (2011). Complete nisin A gene cluster from Lactococcus lactis M78 (HM219853) – obtaining the nucleic acid sequence and comparing it to other published nisin sequences [J]. Genes and Genomics, 33: 217-221.
[7] Wang Cheng. (2010). Construction of an expression vector with Nisin resistant gene nisI as a food grade selection marker for Lactococcus lactis [D]. South China University of Technology.
[8] Ismail Akyol, Ugur Coplekcioglu, Asuman Karakas, et al. (2008). Regulation of the acid inducible rcfB promoter in Lactococcus lactis subsp. lactis. Annals of Microbiology, 58(2): 269-273.
[9] Jacques Edouard Germond, Luciane LaPierre, Beat Mollet, et al. Expression contructs using Lactobacillus delbrueckii subsp. lactis lac repressor protein and its lac repressor binding site, miroorganisms and methods thereof (P). United States Patent: US 6929931 B1. Aug. 16, 2005.
[10] Bolotin A, et al. (2004). Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus [J]. Nat Biotechnol, 22(12):1554-1558.
 "
"